![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
144 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
microscope general structure |

|

|
|
|
SIM |
sulfur indole motility |
j |
|
|
MSA |
manitol salt agar
|
d |
|
|
TSI |
Triple sugar iron agar kligler iron agar |
|
|
|
Citrate |
Citrate polymerase |
|
|
|
phenyls |
d |
|
|
|
CNA |
Colistin and Nalidixic Acid (CNA) |
|
|
|
MR/VP |
Methyl red |
|
|
|
DNAse |
d |
|
|
|
Urease |
Urea hydrolysys |
|
|
|
Simple / Negative |
d |
|
|
|
EMB |
Eoisin Methylene Blue |
|
|
|
Maconckey Agar |
d |
|
|
|
lyser/Orn |
d |
|
|
|
Carbo |
d |
|
|
|
Novo, Baci, Opto |
d |
|
|
|
Oxidase |
d |
d |
|
|
Gram stain |
d |
d |
|
|
Bile |
e |
e |
|
|
General Safey Procedures |
All bacteria are potential pathogens; therefore, always use aseptic techniques. Wipe down your work space with the disinfectant provided before and after lab, wash your hands at the beginning and end of each lab, and always wear your lab coat.Report any spills or accidents to the instructor immediately. Do NOT attempt to clean them up by yourself.Never eat, drink, chew gum, or put anything into your mouth (i.e. pens, pencils, fingers, etc.) while working in the lab.Tie back loose hair.Wear gloves (provided for you) during the laboratory session. |
|
|
|
microscrope Objective Lenses |
4x 10x 40x 100x |
|
|
|
Coccus Sphere |
Pairs and singles chains clusters |
|
|
|
Bacillus (rod) |
Pairs and singles chains Flagellated Bacilli |
|
|
|
Spirochete |
Borrelia Treponema Spirilla |
|
|
|
Differnetial Media |
Differential medium types are those that distinguish microorganisms from one another based on growth characteristics evident when grown on specific medium types. Organisms with differing growth characteristics typically show visible differences in growth when placed on differential media. Examples include blood agar, Eosin Methylene Blue (EMB) agar, Mannitol Salt agar, and MacConkey agar. |
|
|
|
Selective Media |
Selective medium types are formulated to support the growth of one group of organisms, but inhibit the growth of another. These media contain antimicrobials, dyes, or alcohol to inhibit the growth of the organisms not targeted for study. Selective medium types include EMB agar, Mannitol Salt agar, MacConkey agar, and Phenylethyl Alcohol (PEA) agar. |
|
|
|
Media |
Solutions containing all of the nutrients and necessary physical growth parameters for microbial growth.Types:1) chemically defined (synthetic composition known)2) non synthetic (unkown chemical composition)Forms:1) Broth 2) Semisolid 1% agar3) solid more than 1% agar |
|
|
|
culture |
a medium that contains living microbes |
|
|
|
pure culture |
contains a single species |
|
|
|
asceptic |
without contamination |
|
|
|
Asceptic Techniques |
do not perform transfers over any books or papers contamination may occur via droplets & aerosols be organized arange media in advance and label label only on the base of lids place all media tubes in a test tube rack when not in use whether steril or not Take your time |
|
|
|
Broths |
used to grow microbes when fresh cultures or large number of cells are required. Broths of differential media are also used in microbial identification |
|
|
|
agar slant |
used to grow stock cultures that can be refriderated after incubation and maintained for several weeks. Many differential media are agar slants |
|
|
|
Plated Media |
used for obtaining isolation of species differential testing and quantifying bacterial densities |
|
|
|
Transfer to media |
in all cases using media requires asceptic innoculation. |
|
|
|
transfer instruments |
inoculating loops/ needles |
|
|
|
BSL-2 |
Primary concern with these organisms is aerosol production |
|
|
|
Transfer from a broth culture to a sterile broth |
1 label sterile broth tube2 flame inoculating loop3 incinerate lip of the sterile culture4 mix loop in broth 5Remove film from the loop |
|
|
|
agar slant to sterile agar slant |
flame loop & test tube lips zig zag motion |
|
|
|
plate culture to steril broth or agar slant |
use all previous techniques
flame loop
flame lips of any tubes beore and after
tap loop after inoculation always keep loop hand still and move medium
|
|
|
|
From broth From broth |
inoculation from broth medium can be used on Nutrient broth ( broth to broth) Nutrient Agar Slant ( broth to slant) |
|
|
|
from slant |
Inoculation from nutrient agar slant can be used on Nutrient broth ( slant to broth) Nutrient agar slant (slant to slant) |
|
|
|
mixed culture |
culture consiting of two or more species |
|
|
|
pure culture |
containes a single species |
|
|
|
streak plate |
bacteria is always assumed to be a mixed culture. Isolated cells will develop into colonies consiting of only the original cell type Types: quadrant Continuous Zig zag simple T streak pattern |
|
|
|
T streak pattern |
uses only three streaks each occupying a region designated by drawing lines in the shape of a T. One region being larger than the other two Region 1 is the largest streaked with the original sample Region 2 after flaming the loop and allowing to cool the second streak is made by entering the first streak two or three times Region 3 like before the loop is flamed and the streak is taken from region 2 |
|
|
|
Zig zag continuous streak plate |
use zig zag method to streak sterile agar plate |
|
|
|
quadrant streak |
region 1: streak culture back and forth in one quadrant of the plate. stay close to the plates edge
region 2: rotate the plate nearly 90 degrees and touch the in an uninoculated region to cool the loop. Streak again using the same wrist motion. cross into quadrant one Region 3: rotate again 90 degrees streak quadrant using same wrist motion. cross into quadrant two Region 4: streak on last time from quadrant 4 into the center of the loop.
|
|
|
|
Spread Plate |
technique used for isolation which a diluted microbial sample is deposited on a agar plate and spread uniformily across the surface with a glass rod. Step 1: label base of sterile agar plate with name Step 2: arrange alcohol jar and bunsen burned diagnal to each other. Step 3: deposit inoculum volum on the agar surface & dispose of pippeting instrument Step4: flame rod with alcohol Step 5: spread inoculum with steralized rod turining the base of the plate Step 6: return rod to alcohol |
|
|
|
Bacterial Colonies |
Visible Mass of Cells |
|
|
|
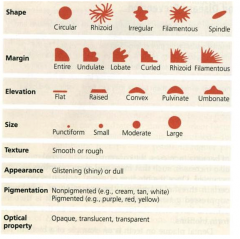
Colony characteristics |
Siize & demnsions
Shape Margin
Surface Appearnce
Texture
Elevations |
|
|
|
Colony Size & Dimensions |
simple measurements such as diameter and length |
|
|
|
Shape |
Coloney shapes may be
Round or Cirucullar
Irregular Punctiform |
|
|
|
Margin |
Margin may be Entire (smooth no irregularities) undulate (wavy) Lobate (lobed) filamentous (unbranched strands) Rhizoid (branched like roots) |
|
|
|
Surface |
surface may be smooth rough wrinkled (rugose) shiny or dull |
|
|
|
Texture |
moist Mucoid (sticky) butyrous (buttery) dry |
|
|
|
Elvations |
Colony may be |
flat raised convex pulvinate (very convex) umbonate (raised in the center) |
|
|
color |
colonies may be opaque ( not see through) transluscent ( light can pass through) |
|
|
|
Agar slants |
used from cultivation and maintenance of stock cultures. Display a variety of growth characteristics Colonies may appear Filiform (dense and opaque with a smooth edge) Friable (dry, crusty) echinulate (spiny) pigmented and other all other colony characterisitcs |
|
|
|
Growth patterns in broth |
Pellicle (floating membrane) Sediment ( skinks to bottom) Uniform ( fine turbidity) flocculent growth (clumps) |
|
|
|
Growth and temperature |
bacteria and archea live in ahbitat ranging from -10 degrees celcius to 110 degrees celcius. Optimum temperature is called the cardinal temeratures |
|
|
|
psychorophiles |
only grow below 20 degrees celcius |
|
|
|
psychotrophs |
cold habitats 0 to 30 degrees celcius |
|
|
|
mesophiles |
live in habitats between 15 and 45 degrees celcius |
|
|
|
Thermophiles |
above 40 degrees celcius |
|
|
|
obligate thermophiles |
will not grow in temperature below 40 degrees celcius |
|
|
|
faculative thermophiles |
will grow below 40 degrees celcius |
|
|
|
extreme thermophiles |
ccan survive 65 to 110 degrees celcius |
|
|
|
simple stains |
consist of a solvent (water or ethanol)
Colored Molecule chromogen
chromophore ( portion of the chromogen which gives it its color)
Auxochrome ( charged portion of the chromogen)
Cells are stained not medium
|
|
|
|
common simple stains |
Methylene blue crystal violet safranin |
|
|
|
negative stains (acidic Stains) |
cannot stand heat fixing
backround is colored not the cell
Have nevatively charged chromogen that is repelled by the negative charged cells.
|
|
|
|
Heat fixing |
kills cells
adheres them to slide
distorts shape |
|
|
|
Gram stain |
differnetial stain which decolorization occurs between the application of two basic stains
The primary Stain & Counter stain
mordant iodine enhances the primary stain usually crystal violet |
|
|
|
The primary stain |
crystal violet
Enhanced by mordant ( Iodine)
Is washed with solvent ( usually alcohol or alcohol acetone mixture) |
|
|
|
The counter stain |
safranin
Used to stain gram negative cells which have lost their crystal violet color after being washed with alcohol/acetone mixture
Gram positive cells cannot be counter stained
|
|
|
|
Gram Negative Cells (redish/ Pink) |
Have an outer membrane
Appear Reddish Pink
Do not retain crystal Violet
Are counter stained by safranin which gives them thier pink/red color
Contain a Higher Lipid content in their cell wall due to the outer membrane.
The solvent disolves the lipid membrane making the gram-negative cell wall more pourous and incapable of retaining crystal violet
|
|
|
|
Gram Positive Cells (violet) |
Do not have a cell membrane Appear violet Retain Crystal violet dye due to their lack of a cell membrane Thicker peptidoglycan cell wall with increased crosslinkage makes them retain crystal violet Do not Counter stain.
|
|
|
|
Acid Fast Stains |
Acid fast stains are used to identify acid fast organisms which have mycolic acids in their cell walls.
Primary Stain: stains cells red Decolarization: acid alohol solvnet Counterstain: Methylene Blue or Brilliant Green |
|
|
|
Mycolic Acid |
Waxy substance that gives acid fast organisms a higher affinity for the primary stain and resistance to decolorization by acid alcohol solution (3% HCl 95%EtOH) repels typical aqeous stains which makes acid fast organisms weakly positive
|
|
|
|
Types of Acid Fast Stain |
Ziehl-Neelsen (ZN)
Kinyoun (K)
|
|
|
|
ZN method |
uses heat as part of the staining process in addition to the heat fixing.
|
|
|
|
kinyoun |
cold stain
heat fixed |
|
|
|
Acid Fast Positive Organisms (Reddish / Pink) |
Contain Mycolic acids in their cell wall
Retain ZN or K stain
Do not decolorize
Do not counterstain |
|
|
|
Acid Fast Negative Organism (Blue /Green) |
Do not contain mycolic acid in their cell wall Do not retain primary stain ZN or K Counterstained with methylene blue or briliant green |
|
|
|
Capsule stain |
Bacterial Capsules are composed of mucoid polysaccharides or polypeptides that repel most stains because of their neutral charge. This technique involves staining the backround with an acidic stain and a basic stain that colorizes the cell. The capsule remains unstained appears as a white halo |
|
|
|
Ensospore Stain |
Used to identify endospore producing bacteria Primary Stain: cells are stained with malachite geen both positive and negative cells will stain Decoloriation: Cells are wahsed with water which removes malchite green in both cells except in the endospores of positive organisms. Counter Stain: both negative and positive organisms will countersain Positive organisms will appear to have a green endospore and a pinkish red cell body. Negative organisms will not have green endospore and will only appear to be pinkish red. |
|
|
|
Endospore positive |
Appear to have green endospore and red/pink surrounding body |
|
|
|
Enodospore negative |
Do not have green endospore Appear entirely red/pink |
|
|
|
Columbia CNA with 5% Sheep blood agar |
Columbia CNA 5%SBA is an undifined differential and selective medium that allows growth of Gram positive Organisms Inhibits growth of Gram negative Organisms Contains B vitamins, casein, beef extract. yeast extract, corn starch, and sheeps blood to provide carbon energy sources. Contain antibiotics Colistin and Nalidixic Acid (CNA) which act against gram negative bacteria. |
Gram Positive Organisms Only |
|
|
Colistin and Nalidixic Acid (CNA) |
disrupt gram negative membranes and interfers with DNA replication |
|
|
|
Columbia CNA 5% SBA Positive Organisms |
Grow well. Well developed colonies are apparent |
|
|
|
Columbia CNA 5% SBA negative Organisms |
Grow Poorely or not at all. Colonies appear faint or do not appear at all. |
|
|
|
Mannitol Salt Agar (staphylococci) |
Differentiates Staphylococcus aureus from other staph species. Differential/Selective Medium, which contains the carbohydrate Manitol and Sodium Chloride (7.5%). Also contains pH indicator phenol red. Used to detect fermentation and growth of viable organisms. Mannitol provides the substrate for fermentation and sodium chlordide makes the medium selective. Most Staph. Grow on MSA but do not ferment growth appears pink or red. Fermenting Staph. Appear with yellow halo. changes the pH of the medium. |
|
|
|
Positive MSA organism |
Staphylococcus Aureus
Fermenting organism that Grows with yellow halo |
|
|
|
Negative MSA Organism |
Other Staphylococcus species Do not ferment and do not change the color of the medium. |
|
|
|
MacConkey Agar (lactose Fermentation) |
Selective/Differential Medium.
Used to differentiate members of the Enterobacteriaceae based on the ability to ferment lactose.
Contains lactose, bile salts and crystal violet that inhibit Gram positive bacteria.
Organisms that ferment lactose lower the pH and their colonies turn pink to red. |
inhibits gram-positve bacteria |
|
|
MacConkey Positive Organisms |
Gram negative Enterobacteria that Ferment Lactose
Colonies appear red/pink Pink to red Bile may precipitate |
|
|
|
MacConkey Agar Negative Organisms |
Gram-postive Organisms will not grow Non-lactose fermentors no color change appears natural |
|
|
|
Eosin Methylene Blue Agar (EMB) (lactose fermentation) |
Selective/Differential Medium. Contains Digest of gelatin, lactose, and dyes eosin Y and methylene blue. Differentiates vigorous lactose fermentors. which turn the colony dark purple or black or dark green. Dark growth is characteristic of Escherichia coli and is accompanied by green metalic sheen. Less aggressive Lactose Fermentors produces colonies that range from pink to dark purple. Lactose non fermentors reatain their natural color. The dyes inhibit gram postive bacteria (except Enterococcus and Staphylococcus) |
inhibits gram positve bacteria |
|
|
EMB postive Organisms ( Agressive Lactoses Fermentors) |
Escherichia coli Dark purple black colonies Green metalic sheen |
|
|
|
EMB postive Organisms ( Non Agressive Lactoses Fermentors) |
Enterobacter or Klebsiella Produce colonies that range from pink to dark purple. |
|
|
|
EMB Negative Organisms (non lactose fermentors) |
Do not form dark colonies
Take on the color of the medium
Gram postive bacteria do not grow
|
|
|
|
Differential Testing |
Types: Energy Metabolism (fermentaion of carbohydrates and aerobic and anaerobic resperation) Utilization of a specified medium component Decraboxylation and deamination of amino acids Hydrolytic reactions requiring intracellular or extracellular enzymes Multiple Reactions performed in a sing combination medium Atimicrobial susceptibility Miecellaneous differential tests |
|
|
|
Energy and maetabolism |
r |
r |
|
|
Phenol Red Fermentation Broth |
Differential fermentation medium. composed of standard ingredients to which a single carbohydrate is added. Tests for an organisms ability to ferment that particular carbohydrate. Contains peptone and the pH indicator phenol red. Identifies end products of fermentation. Can be used to differentiate members of enterobacteriaceae. Distinguish them from other gram positive fementors such as streptococcus and lactobacillus species. Fermentation is indicated by the production of gas products and the change in phenol red. (inverted dunham tube) Phenol red is yellow below pH 6.8 Phenol red is red inbetween 6.8 and 7.4 Phenol red is pink to magena above pH 7.4 Production of ammonia raises the pH and turn the broth pink if no acid is prodced or if acid production has ceased to the consumption of the carbohydrate. |
|
|
|
Methyl Red and Voges-Proskauer (MR-VP) Fermentation of glucose |
MR-VP broth is a combination medium used for both methyl red and Voges proskauer tests.
The MR test is designed to detect organisms capable of performing a mixed acid fermentation which overcomes the phophate buffer in the medium and lowe the pH. Succinate is produced form the addition of carbon dioxide to phosophenolpyruvate
whereas the other end products are derived from the reduction of pyruvate to lactate or its oxidation to acetyl-CoA and formate.
Conversio of CoA to acetate results in the formation of one ATP and it can be reduced to ethanol.
Formate can further be reduced down into Hydrogen gas and Carbon dioxide. the acids produced by these organism are stable.
Acids produced by other organism can be conveted to more neural products or their fementation shifts to produce more neutral products in rsponse to the lowered pH.
The Voges proskauer test identifies organisms that are able to ferment glucose, with the production of acetoin and 2,3, butanediol, which have a neutral pH. |
|
|
|
MR-VP postive organisms |
MR postive organisms will change the medium color to red immediately VP postive organisms will change the medium red within 60 minutes. |
|
|
|
MR-VP negative organism |
MR negative orgnism will not change the medium color at all VP negative organisms will not change the medium red within 60 minutes. |
|
|
|
Citrate utilization test |
Determines the persence of citrate permease used to differentiate members of enterobacteriaceae that have the ability to ferment carbohydrates and they also have the ability to aerobically respire which means they have a functional citric acid cycle
Citrate postive bacteria hydrolyze citrate into oxaoacetate and acetate using the enzyme citrate lyase. From ther oxaloacetate is decarboxylated tp pyruvate simultanesouly using the enrgy released to pump oxaloacetate/pyruvate into the cell. |
|
|
|
Citrate utilization positve organisms |
Slant agar becomes blue (citrate permease is present) No color change but growth is apparent (citrate permease is peresent) |
|
|
|
Citrate utilization negative organisms |
No color change and no growth (citrate permease is not present) |
|
|
|
Triple Sugar Ion Agar/ Kligler Iron Agar |
TSI is a rich medium designed to differentiate bacteria on the basis of sulfur reduction and glucose, lactose, sucrose fermentation. Contains anaimal proteins as sources of carbon and nitrogen, ferrous sulfate, sodium thiosulfate.Phenol red indicates pH And ferrous sulfate is the hydrogen sulfide indicator. organisms that are able to ferment glucose and lactosr or sucrose turn the medium yellow. |
|
|
|
TSIA Results
Yellow slant and yellow butt |
glucose and lactose fermentation with acid accumulation in butt |
|
|
|
KIA results Yellow butt and yellow slant |
Glucose and lactose/sucrose fermetnation with acid accumilation |
|
|
|
TSIA/ KIA Results
Red slant and yellow butt |
glucose fermetnation with acid production,
Proteins catabolized aerobically with the alkaline products |
|
|
|
TSIA/ KIA Results Red slant and red butt |
No fermentation peptone catabolized aerobically and anaerobically with alkaline products, isolate is notr from enterobacteriaaceae |
|
|
|
TSIA/ KIA Results Red slant no change in butt Red slant no change in butt |
no fermentation peptone catabolized aurbically with alkaline products isolate is not from enterobacteriaaceae no fermentation peptone catabolized aurbically with alkaline products isolate is not from enterobacteriaaceae no fermentation peptone catabolized aerobically with alkaline products isolate is not from enterobacteriaaceae |
|
|
|
TSIA/ KIA Results no vissible changes |
organism is growing slowly or not at all isolate is not from enterobacteriaceae |
|
|
|
TSIA/ KIA ResultsBlack precipitate in the agar |
sulfur reduction |
|
|
|
TSIA/ KIA Results Cracks in Agar |
Gas production |
|
|
|
Catalase Test |
Used to determine bacteria that produce catalase The electron transport chains ETC of aerobic and facultatively anaerobic bacteria are composed of molecules capable of accepting and donating electrons as condition dictate. |
|
|
|
Catalase Test positive Organism |
apparent bubbles in gas production when put into hydrogen peroxide |
|
|
|
Catalase negative test |
no bubbling or gas production when placed in H2O2 |
|
|
|
Oxidase Test |
Test for the presence of cytochrome C Differntiates Faculative anerobes from non faculative anerobes |
|
|
|
Oxidase test postive organism |
Poduces a color change within seconds if the reducing agent become oxidised Cytochrome C is present |
|
|
|
Oxidase test Negative organism |
no color change no cytochrome c is present in the medium |
|
|
|
Amino Acid Decarboxylation
Fermentation of amino acids Decraboxylation amino acid s |
contains peptone, glucose, the pH indicator bromcresol purple an coenzyme pyridoxal phosphate |
no color change: no decarboxylation
Yellow: fermentation no decarboxylation
Purple: decarboxylation |
|
|
phenylalanine deaminase test |
differentiates organisms that produce phenylalanine deaminase can be identified by their ability to deaminase the amino acid phenylalanine |
Green color: phenylalanine deaminase is present No color change: phenylalanine deaminase is not present |
|
|
Urea hydrolysis (urease test) |
tests for an organisms ability to hydrolyze urea |
pink: rapid urea hydrolysis urease production Orange or Yellow: No urea hydrolysis |
|
|
DNA Hydrolysis Dnase Test |
Tests for an organisms ability to produce deoxyribonuclease DNase |
clearing in agar loss of green color: DNase is present No clearing in Agar around growth: DNase is absent or not detectable. |
|
|
Bacitracin |
used to differentiate streptococci from staphylococcus |
Streptococci is susceptible and a ring will appear around the A disk Staphylococcus Is resistant and no ring will appear |
|
|
Novobiocin |
used to differentiate coagulase negative staphylococci.
used to identify novobiacin resistant staphylococcus saprophyticus
|
Growth should remain around the disk |
|
|
Optochin |
used differentiate streptococcus pneumonia for other alpha hemolytic streptococci |
growth should not occur around p disk |
|
|
Bile Esculin Test BEA |
used for the isolation and identification of bile esculine positive enterococci and the Boris group of streptococci from non group D streptococci |
Medium is darkened within 48 hours: organism hydrolyzes esculent in the presence of bile, indicates group D streptococci No darkening of the medium within 48 hours: organism does not hydrolyze esculent in the presence of bile or does but not in detectable amounts; presumptive determination as a non-Group D streptococcus or entercoccus |
|
|
Blood Agar Hemolysis |
Used to identify gram postive cocci that produce hemolysins Alpha Beta Gamma |
Alpha Hemolysis: characterized by a green zone around colonies Beta hemolysis: clear Halo Clearing around he growth is a result of complete lysis of red blood cells Gamma Hemolysis: No halo around colonies |
|
|
Sulfur Indole Motility (SIM) |
Used to identify bacteria that reduce sulfur produce indoles and are motile |
|
|
|
Sulfur postive SIM |
Black color in the medium
cannot determine motility |
organism respires anaerobically with sulfate as the final electron acceptor or hydrolyses the Amino acid cysteine |
|
|
Indole postive SIM |
Red ring in top layer. |
organism produces and hydrlyses tryptophan into indole and pyruvate |
|
|
Motile Positive SIM |
Growth radiating outward from the stab line |
|
|
|
obligate aerobes
|
Obligate aerobes need oxygen because they cannot ferment or respire anaerobically. They gather at the top of the tube where the oxygen concentration is highest. |
|
|
|
micro-aerophiles |
Microaerophiles need oxygen because they cannot ferment or respire anaerobically. However, they are poisoned by high concentrations of oxygen. They gather in the upper part of the test tube but not the very top. |
|
|
|
facultative anaerobes |
both anaerobic and aerobic Grow primarily at top of tube but spreads throughout tube |
|
|
|
aerotolerant |
anaerobic but occurs in the presence of oxygen found all over tube. |
|
|
|
morphology |
|

