![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
777 Cards in this Set
- Front
- Back
|
What is a common antecedent on Guillan Barre?
|
Camplyobacter jejuni
|
|
|
Describe color staining of gram positive and gram negative bacteria. Explain structural differences b/w the two.
|
Gram positive turns purple with crystal violet. Has 40 sheets of PDG (thick layer)
Gram negative turns red with safranin stain. 1-2 sheets of PDG (thin layer) |
|
|
How does salmonella typhi present?
|
fever, diarrhea, headache, rose spots on abdomen.
|
|
|
Name components of a gram negative bacteria membrane and their importance.
|
(from in to out)
1. Phospholipid 2. Cytoplasmic Membrane 3. PDG 4. Phopholipid 5. Proteins & Porins (spanning membrane) 6. LPS -- endotoxin that also acts as a superantigen. 7. Core polysacc. 8. O-polysacc This thick layer is a reason for G-'s antibiotic resistance. |
|
|
What are the arenaviruses?
|
LCMV -- lymphocytic choriomeningitis virus (presents similarly to congenital toxoplasmosis)
Lassa fever encephalitis |
|
|
Which bug is transmitted in pet feces and can mimic Crohn's disease or appendicitis?
|
Yersinia enterocolitica
Cold growth, outbreaks of diarrhea often in daycare centers |
|
|
What is PDG?
|
A sugar polymer cross-linked w/peptide polymers (N-acetylglucosamine, N-acetylmuramic acid). The muramic acids also have bonds with each other. Easily cleaved.
|
|
|
Which question-mark shaped bacteria is found in water contaminated with animal urine? How does it present?
|
Leptospirosis includes flulike sympyomes, fever, headache, ab pain, jaundice, photophobita with conjunctivitis.
Wil's disease -- severe from with jaundice and azotemia from liver and kidney dysfunctionl fever, hemorrahe, and anemia |
|
|
What are the components of G- bacteria? Where is the interbridge?
|
D-ala, L-ala, D-glu, DAP
The interbridge is located b/w I-Lys and the 5 Gly chain. |
|
|
Which diarrheal protozoa is acid fast?
|
cryptosporidium
|
|
|
Where is the interbridge on a G+ bacteria?
|
B/w DAP and D-Ala
|
|
|
How to treat toxoplasma gondii?
|
sulfadiazine + pyrimethamine
|
|
|
What is vancomycin?
|
It acts on D-ala to lactate it so it cannot form an interbridge.
|
|
|
How to treat entamoeba histolytica?
|
metronidazole and iodoquinol
|
|
|
How does penicillin work? How is it resisted?
|
It inhibits murein synthesis. B-lactamase, carried by plasmid, breaks down penicillin, leading to resistance.
|
|
|
How to treat t. brucei (african sleeping sickness)
|
Suramin for blood borne
Melarsopeol for CNS penetration |
|
|
What are archaebacteria?
|
They live in harsh environment and lack a PDG layer
|
|
|
how to treat t. cruzi?
|
nifurtimox
|
|
|
How are spores utilized? What are they made of? What types of bacteria produce spores?
|
Spore formation occurs under stress as a survival mechanism. Spores have 2 PDG layers and a keratin-like coat.
Some G+ bacteria (bacillus, clostridium) are spore-forming. |
|
|
How to treat Leishmania?
|
Sodium stibogluconate
|
|
|
What are the stages of binary fission?
|
1. DNA Replication
2. Cell Elongation 3. Septum Formation 4. Completion of Septum w/ Formation of Distinct Walls |
|
|
How to treat babesia? How does babesia present?
|
fever and hemolytic anemia; predom in NE US (maltese cross)
guinine, clindamycin |
|
|
What are the growth phases for bacteria? Describe them. How is this related to viable count and optical density?
|
1. Lag phase -- move bacteria from one place to another, has to adapt.
2. Exponential -- division and proliferation 3. Stationary -- limiting factor stunts growth 4. Death Viable count is the exact number of bacteria in initial tube. Optical density is a measure of turbidity (particles in solution). |
|
|
What are the nematodes? What are the intestinal nematodes? What are the tissue nematodes?
|
Round worms
Intestinal: enterobis vermiularis, ascaris lumbricoides, trichenella spiralis (eaten) stronglyoides, ancylostoma, necator (penetrate skin) Tissue: dracula, onchocera, lao lao, wuchereria bancrofti, toxocara canis |
|
|
Compare eukaryotes and prokaryotes.
|
1. Euk -- Diploid
Prok -- Haploid (single chromosome) 2. Euk -- linear chrom's Prok -- single circular chrom 3. Euk -- nucleus Prok -- no nucleus 4. Euk -- intons Prok -- nope 5. Euk -- mitochondria & chloroplasts have a self-replicating double stranded DNA molecule Prok -- extrachromosomal plasmids that can replicate Prok genes code for 1 products only. |
|
|
How does trichenella spiralis present?
|
undercooked meat, usually pork; inflammation of the muscle, periorbital edema, myocarditis.
|
|
|
What is the gene structure of a bacteria?
|
1. Promoter (-35)
2. Transcription Initiation Site (-10) 3. Shine Delgarno Ribosome Binding Site (GC rich) 4. Start Codon/ATG (+1) 5. Stop Codon 6. Termination (inverted repeats and uracils) |
|
|
What is stronglyoides associated with? How to treat?
|
intestinal infection, vomitting, diarrhea, anemia, a/w HTLV-1
treat with bendazoles or pyrantel pamoate |
|
|
What is a core enzyme? What is a holoenzyme?
|
1. a holoenzyme is a complex w/2 alpha subunits, 1 beta subunit, 1 beta prime subunit, 1 sigma subunit
2. a core ezyme is missing the sigma subint. It can transcribe but cannot recognize promoters. Therefore, the sigma subunit is involved with promoter recognition. |
|
|
How does drancunculus medinesis present? How to treat?
|
In drinking water, skin inflammation and ulceration
treat with niridazole |
|
|
What are the individual jobs for each of a holoenzyme's subunits?
|
1. sigma -- recognize promoter sequence on DNA
2. beta' -- recognize & bind DNA 3. beta -- binds NTP, interacts with sigma 4. alpha -- essential for assembly & activation of enzyme by regulatory proteins |
|
|
How to treat loa loa, wucheria bancrofti, and toxocara canis?
|
diethylcarbamazine
|
|
|
What types of sigma factors exist?
|
1. sig 70 -- basally active, the principal sigma
2. sig 32 -- heat shock 3. sig 60 -- nitrogen starvation sigma subunits are interchangeable. |
|
|
How does toxocara canis present?
|
food contaminated with eggs; causes granulomas (if in retina --? blindness) and visceral larva migrans
|
|
|
Describe Initiation.
|
First stage of transcription.
1. DS DNA w/promoter region 2. sig factor comes and sits on promoter region 3. core enzyme assembles 4. holoenzyme forms, transcription is initiated. once transcription begins, sig can dissociate from DNA as it is not no longer needed 5. core enzyme continues along to make mRNA from template stanf until it reaches termination site 6. core enzyme can (if it wants) dissociate |
|
|
What are the cestodes? Which one causes B12 deficiency? Which one can cause anaphylaxis upon cyst bursting?
|
Tanea solium (cysticercosis)
Diphyllobothrium latum (from fish, B12 def) Echinocossus (eggs in dog feces can cause cysts in liver, causes anaphylazis if echinococcal antigens are released from cysts -- surgeons inject ethanol before removal to kill daughter cysts) |
|
|
Describe Elongation.
|
Second stage of transcription.
1. RNA Polym. adds nucleotides to match base pairs on template strands --> RNA polym. is not as accurate as DNA polym., but doesn't need to be b/c are many copies of a given protein and mRNA around 2. RNA polym. adds at a rate of 20-50 bases/s; slower in G-C region, faster in A-T region 3. topoisomerase precedes and follows polymerases to relieve supercoiling of DNA |
|
|
What are the trematodes? How to treat? Which organism is from crab meat?
|
schistosoma
clonorchis sinesis paragonimus westermani (undercooked crab meat; causes inflammation and 2ndary bacterial infection of lung, causing hemoptysis) treat with praziquantel |
|
|
Describe Termination. Describe both pathways.
|
Third stage of transcription. There are two pathways: Rho dependent, Rho independent.
1. Dependent -- Rho protein binds to mRNA, slides along transcript until it reaches RNA polym. It is able to "catch up" to poly. b/c termination is slower at the G-C end. DNA will be kicked off/ 2. Independent -- involves formation of a stem-and-loop structure and depends also on slowing of elongation sequence. Depends on A-T rich region that destabilizes elongation complex. a) RNA polym. slows at termination site b) inverted repeat in RNA sequence folds c) stem-and-loop structure forms d) disruption of RNAP complex. |
|
|
What are the live attenuated vaccines?
|
smallpox
yellow fever chicken pox Sabin's MMR |
|
|
How do transcription and translation work in bacteria?
|
Simultaneously.
1. helicases/topoisomercases unwind DNA 2. RNAP w/ sigma starts transcription 3. RNAP makes mRNA 4. as mRNA emerges, ribosomes bind to Shine-Delgarno seq. and start translation |
|
|
What is the only DNA virus that replicates in the cytoplasm?
|
Pox
|
|
|
What is tRNA? How is it structured?
|
It is needed for translation. It has an amino acid attached to 3' end (determined by anticodon) and on other side, anticodon that binds codon on mRNA
|
|
|
What are the +ssRNA?
|
retro
toga flav corono hepe calci picorna |
|
|
What does translation need?
|
1. ribosomes
2. mRNA 3. tRNA 4. aminoacyle-tRNA synthase 5. L-amino acids 6. ATP |
|
|
What is the only dsRNA?
|
Reo
|
|
|
What is the mechanism for translation?
|
1. small ribosomal subunit recognizes mRNA binding site (Shine-Dalgarno seq)
2. recognizes start codon 3. met-tRNA binds to complex 4. large subunit binds and closes complex 5. aminoacyl-tRNA fit into hollow chamber and slide along mRNA (the A site) 6. translation starts at P site and protein exits at E site as it is pushed by other a-TRNA through the A site 7. at termination, release factor comes in at stop codon, ribosome falls off, protein freed |
|
|
What are the naked viruses?
|
Calci
Picorna Reo Pap Adeno Parvo |
|
|
What is an operon?
|
A set of genes needed by bacteria physiology altogether at a certain point in time. It controls the expression of a gene by influencing which seq. will be transcribed into mRNA.
e.g. we have 5 genes, 3 are needed for usage of lactose as carbon source; put them together w/promoter, can be regulated together: 1 long mRNA w/promoter and 3 ORF & term. Will be activated based on amount of lactose in environment |
|
|
Where do DNA and RNA viruses replicate?
|
All DNA viruses replicate in the nucleus (except pox -- cytoplasm)
All RNA viruses replicate in the cytoplasm (except influenxa and retro -- in nucleus) |
|
|
What is a polycistronic operon? How does it compare to a monocistronic operon?
|
Has one promoter for all ORF.
e.g. we'll end up with 2 proteins from the same mRNA (but not same DNA region), regulated by same promoter. Monocistronic operons involve use of weak and strong promoters at the same time. Same regulation of promoters with use of weak/strong sig factors, end up with genes with their own promoters. |
|
|
What are the Picornaviruses?
|
Polio
Enter Echo Coxsackie Rhino HAV |
|
|
What are ways in which a gene can be regulated?
|
1. DNA methylation -- marking own DNA in order to recognize foreign and degrade (by restriction enzyme), using material as energy. This could cause problems for transcription (b/c of RE)
2. Effectors bind to allosteric site on enzyme and block substrate from the binding site; or it is needed to enable substrate binding |
|
|
What are the enteroviruses?
|
Polio
Entero Cox HAV |
|
|
Explain effectors in detail.
|
They regulate association to form RNAP and affect it:
1. a different protein binds to promoter region, eliminating RNAP and sig factor (blocks) 2. protein causes sig factor to have higher recognition ability (enhances) |
|
|
Which viruses acquire their nuclear envelope from nuclear membrane?
|
Herpes
|
|
|
What are repressors?
|
Involved in negative regulation, bound to an operator.
An effector binds the repressor, which in turn can no longer bind to operator, turning gene on. OR Repressor can only bind for effector is present. Its removal inactivates repressor, cannot block operator |
|
|
How does adenovirus present?
|
Febrile pharyngitis -- sore throat, acute hemorrhagic cystitis
Pneumonia Conjuntiva |
|
|
How does positive regulation work?
|
Getting RNA polymerase to sit on operator. Removal of ligand will disable inducer, activator can bind to DNA. Hooray.
|
|
|
What is the number 1 fatal cause of diarrhea in children?
|
Rota
|
|
|
How does the lac operon work?
|
It is a repressor protein under negative contort. Control expression of 3 structural genes for lactose metabolism.
1. Lac repressor binds operator 2. lactose is inducer, binds repressor 3. RNAP transcribes genes |
|
|
What are the segmented RNA vruses?
|
Reo
Orthomyxo Bunya Arena |
|
|
How does the arg operon work?
|
It is a repressor protein that contols expression of genes necessary for arginine metabolis,
Expression is blocked by presence of co-repressor argining, which binds to repressor and binds it to operator |
|
|
What are the most common causes for aseptic meningitis?
|
Entero (Echo)
HSV2 VZV mumps HIV LCMV |
|
|
How does the trp operon work?
|
To avoid tryptophan toxicity.
Repressor protein is activated by increased intracellular concentration of tryptophan to prevent trascription. Translation will be atenuated by formation of mRNA stem loop/ |
|
|
What are the flavi viruses?
|
HCV
Yellow fever Dengue St. Louis encephilitis West Nile |
|
|
What is CAP?
|
Catabolism activator protein. If enough energy in cell, can spend some on processing lactose and if not, preferentially use glucose, shut down lac operon.
cAMP + CAP will activate lac operon if lactose is cround. |
|
|
What are the togaviruses?
|
Rubella
WEE VEE EEE |
|
|
What is recombination? What types are there?
|
Incorporation of DNA into chromosomes.
Homologous -- regulated by RecA, legitimate Nonhomologous -- transposons, bacteriophages, illegitimate |
|
|
What are the paramyxo viruses?
|
Roseola (measlease)
Mumps Parainfluenza (croup) RSV (bronchiolitis in babies) |
|
|
What is a transposon?
|
t is a moveable genetic elementl changes position of genes on strang. Can move on same chromosome or hop into plasmid (and back). Can encode virulence genes and/or antibiotic resistance.
|
|
|
What are the filoviruses?
|
Ebola/Marburg
|
|
|
What is Transformation?
|
Direct uptake and recomb. of naked DNA fragments by competent bacteria. Can be induced in lab by calcium chloride, heat, electrick shock.
H. Influenza, S. Pneumoniae, Bacillus, Neisseria |
|
|
What are competent bacteria?
|
These produce competence-specific proteins. These are membrane-associated DNA binding proteins. Have cell wall autolysin and nucleases. In bacillus and strep, competence is regulated by quorum-sensing mechanisms (cell-density dependent).
Bacil -- 20% become competent, stay that way for few hours Strep -- 100% become comp, stay that way for few minutes |
|
|
What are the bunyavirus?
|
California encephilitis
Sandfly/rift valley fever Crimean-Congo hemorrhagic fever Hantavirus -- hemorrhagic fever, pneumonia |
|
|
What is the mechanism of transformation?
|
1. free DNA binds to membrane DNA-binding proteins
2. passage of one of the strands into cell whil enuclease activity degrades other 3. single strand bound by specific proteins and recmbin w/ homologous regions mediated by RecA protein occurs |
|
|
What are the -ssRNA?
|
(brings its own RNA-dependent RNA polymerase_
Arena Bunya Paramyxo Ortho Filo Rhabdo |
|
|
What is transduction?
|
Phage-mediated transfer of host-DNA.
|
|
|
How does yellow fever present?
|
transmitted by Aedes mosquito
high fever, black vomit, hemorragic disease, and jaundice |
|
|
What is a temperate phage?
|
Remains dormant as a prophage, then various activation signals (like UV) trigger prophase to enter lytic cycle. C1 represses transcription but can be degraded by UV
|
|
|
What are the common causes of conjunctivitis?
|
H. influenza
Adeno S. pnuemoniae |
|
|
Explain conjugation.
|
In G-, DNA is transferred via conjugative plasmid (F pilus) to recipient cell by physical contact.
In G+ cell, plasmids are transferred not through a pilus but through contact b/w two cells, induced by quorom-sensing. R factor is a plasmid that contains drug resistance gene. |
|
|
What are common causes for the common cold?
|
Rhino
Corona Adeno Influenza C Cox |
|
|
What is the life cycle of trypanosome?
|
1. parasite in host; long, slender form
2. in bloodstream; short, stumpy form 3. taken up by tsete fly; forms procyclic form which multiplies in gut 4. grows into eptmastigote form in fly's saliva 5. epimastigote --> metacyclic form 6. new bite will infect animal Parasite also has a VSg on its surface, leading to evolution of parasite so that it can propogate again in genetically modified vectors. |
|
|
What are the functions of HA and NA?
|
HA -- promotes viral entry
NA -- promotes viral release |
|
|
How do parasitic protazoa survive under harsh conditions?
|
many develop into cysts, which are less metabollically active. The cyst is surrounded by a thick external cell wall, capable of protecting. Can stay in cyst form until environment is more favorable. Parasites that cannot/do not form cysts rely on direct transmission from host to host
|
|
|
What do paramyxoviruses contain? How is it targeted in pharmacotherapy?
|
All contain surface F (fusion) protein, which causes respiratory epithelial cells to fuse and form multinucleated cells.
Palivizumab is used in RSV to neutralize F protein |
|
|
How can an intracellular parasite cause death of the host?
|
1. competing with host for nutrition
2. lysing host cells 3. immunosuppression 4. inflammation |
|
|
In HIV, where do mutations often occur?
|
env (gp 120, gp 41)
|
|
|
How can an extracelular parasite cause death of the host?
|
1. nutrition competition
2. damaging tissues (e.g. penetration) 3. bleeding 4. killing normal flora 5. blocking tracts 6. immunosuppression 7. inflammation |
|
|
Which bacteria show antigenic variation?
|
Neisseria (pili)
Strep (M protein) Mycoplasma Lyme |
|
|
What are methods of bacteria that cause disease?
|
1. Adhesion
2. Colonization -- some virulent bacteria produce special proteins, allowing them to colonize e.g. H pylori using urease to survive in low pH 3. Invasion -- produce proteins that either disrupt host cell membranes or stimulate endocytosis into cell 4. Immune Response Inhibition -- block receptors on maccrophages, which bind to opsonized pathogens, affecting antigen presentation 5. Toxins -- endotoxins, exotoxins |
|
|
Which protozoa show antigenic variation?
|
T. brucei (afircan sleeping sickness)
Plasmodium falciparum |
|
|
Order these from high virulence to low virulence:
Salmonella Shigella Listeria |
Shigella
Salmonella Listeria |
|
|
Which viruses show antigenic variation?
|
Influenza
HIV (env) Flavi (E protein) |
|
|
What are endotoxins?
|
Located on G- bacteria in the LPS. Lipid A is the toxic portion (part of cell membrane).
Causes hyperepsoniveness (nonspecific activator), attracting macrophages, cytokines, etc. Not denatured by boiling, doesn't form toxoid, low degree of activity and specificity. |
|
|
What HIV associated infections occur when CD4 < 50
|
CMV retinitis and esophagitis
disseminated M avium-intracellulare cryptococcal meningioencephalitis |
|
|
What are exotoxins?
|
Produced by either G- or G+, secreted from bacteria. Denatured by boiling, forms toxoids, high degree of activity and specifictiy
|
|
|
With ToRCHeS infections, what are the common nonspecific signs?
|
hepatosplenomegaly, jaundice, thrombocytopenia, and growth retardation
|
|
|
What is the complement system?
|
C1 - C5; trigger recruitment of inflammatory cells. Tag pathogens for destruction; disrupt plasma membrane of infected cell, resulting in cytolysis of infected cell, causing death of pathogen. Clear body of neutralized antigen-antibody complexes.
|
|
|
ToRCHeS:
Toxoplasma |
chorioretinitis
hydrocephalus intracranial calcifications |
|
|
Toxin mechanism of C. Diptheriae
|
Diptheria toxin targets the heart, nerve, epithelium.
ADP ribolyzation of EF 2, leading to protein synthesis inhibiton |
|
|
ToRCHeS:
rubella |
PDA
cataracts deafness (and/or blueberry muffin rash) |
|
|
Toxin mechanism of P. Aeruginosa
|
Exotoxin A targets liver.
ADP ribolyzation of EF 2, leading to protein synth inhibition |
|
|
ToRCHeS:
CMV |
hearing loss
seizures petechial rash |
|
|
Toxin mechanism of S. Dysenteria
|
Shiga toxin -- enzymatic ally cleaves rRNA, interferes w/60s, inhibiting protein synth
|
|
|
ToRCHeS:
HIV |
recurrent infections
diarrhea |
|
|
Toxin mechanism of V. Cholerae
|
ADP ribolyzation of G peoteins stimulates adenylate cyclase, increases cAMP in GI tract = continual secretion of water and electrolytes
|
|
|
ToRCHeS:
HSV |
temporal encephalitis
herpetic (vesicular) lesions |
|
|
Toxin mechanism of ETCT E. Coli
|
ADP riboly of G proteins stimulates guanulylate cyclase, promotes secretion of water and electrolytes from intestinal epithelium
|
|
|
ToRCHeS:
Syphillis |
often stillbirth, hydrops fatalis
presents with structural abormalities (notched teeth, saddle nose, saber shins, short maxilla) |
|
|
Toxin mechanism of EHCH E. Coli
|
interferes w/60s, inhibiting protein synth
|
|
|
Which antibiotics treat gram negative only?
|
Monobactam (aerobic)
Aminoglycosides (aerobic) Polymyxin |
|
|
Toxin mechanism of B. Anthracis
|
Edema factor is adenylate cyclase that causes increased levels in intracellular cAMP in phagocytes and formation of ion-permeable pres in membrane. Decreased phagocytosis, causes edema, kills cells
|
|
|
Which antibiotics treat gram positive only?
|
Vancomycin
Clindamycin Linezolid |
|
|
What are the toxicities of methicillin?
|
interstitial nephritis
|
|
|
Toxin mechanism of B. Pertussis
|
Increased cAMP in phagocytes, hemolysis. ADP ribosylates G
|
|
|
What are the toxicities of vancomycin?
|
Nephrotoxicity
Ototoxicity Thrombophlebitis Red Man Syndrome |
|
|
Sulfonamides antagonize what compoud? What is their mech?
|
PABA
PABA is needed to synth folate de novo. Sulfonamides inhibit dihydropteroic acid synthase (only in bacteria) to prevent synthesis of tetrahydrofolic acid. |
|
|
Toxin mechanism of C. Tetani
|
Tetanus toxin is a neurotoxin. Zn2+ dependent protease that blocks release of inhibitory transmitters glycine & GABA = spastic paralysis
|
|
|
Why are sulfonamides contraindicated in neonates?
|
displace bilirubin from albumin, causing kernicterus
|
|
|
Toxin mechanism of C. Botulinum
|
Botulism toxin is Zn2+ dependent protease inhibiting release of Ach = flaccid paralysis
|
|
|
How does TMP work? What are the toxicities? How can they be alleviated?
|
inhibits bacterial dihydrofolate reductase.
megaloblastic anemia, leukopenia, granulocytopenia. alliviate with supplemental folinic acid (leucovorin rescue) |
|
|
Toxin mechanism of C. Aures
|
Superantigen. Toxic Shock Syndrome. Enhances endotoxins in gut normal flora, leading to toxic manifestation of LPS. Shock, capillary leakage.
omg tampons are scary. |
|
|
How is TMP-SMX used?
|
recurrent UTI
Shigella Salmonella PCP |
|
|
What are the cell receptors for the following viruses?
1. Measles 2. Herpes 3. Rabies |
1. CD46
2. Herparin Sulfate 3. Nicotinic Ach receptor |
|
|
Penicillins are synergistic with what other antibiotic drug class in the treatment of enterococcal and pseudomonal infections?
|
aminoglycosides (cannot easily cross cell wall). Inhibition of cell wall formation = easier entrance
|
|
|
What are the targets for the following viruses?
1. HIV 2. EBV 3. Reovirus 4. Rabies 5. Common cold 6. Flu |
1. Th cells, macrophages, microglia; uses receptor CD4
2. B cells; uses CD21 3. Neurons; uses beta-andregenic receptors 4. Neurons; uses Ach receptors 5. ICAM 1 6. Sialic acid residues |
|
|
What are the three b-lacatmase inhibitors that can be used in combination with pennicillins?
|
1. Clavulanate
2. Sulbactam 3. Tazobactam |
|
|
Throughout the evolution bacteria preserved the ability to produce and secrete proteolytic enzymes in order to:
|
Degrade proteins in the surrounding as a source of nutrients
|
|
|
What is the mech of methacillin resistance in s. aureus?
|
production of an alternative PBO 2a
|
|
|
3.An outbreak of sepsis caused by Staphylococcus aureus has occurred in the newborn nursery. You are called upon to investigate. According to your knowledge of normal flora, what is the MOST likely source of the organism?
|
Nose
|
|
|
What are the AG antibiotics?
|
gentamicin
tobramycin streptomycin neomycin amikacin |
|
|
Endospores are characterized by...?
|
1. A lack of metabolic activity
2. Greater resistance to drying than vegetative cells 3. Multiple covering layers 4. Occur in both aerobic and anaerobic positive bacillus |
|
|
What is the mech for clindamycin?
|
binds to 50 ribosomal subunit to inhibit translocation of peptidyl-RNA, inhibiting protein synthesis
|
|
|
1.Staphylococcus aureus causes the following diseases EXCEPT:
A.Pneumonia B.Endocarditis C.Food Poisoning D.Cellulitis E.Bacterial overgrowth syndrome |
Cellulitis
|
|
|
What is the mechanism of action of macrolide antibiotics? What are they used for? What are the side effects?
|
binds to 50s ribosomal unit to inhibit translocation
ATYPICAL PNEUMONIAS prolonged QT interval w/erythromycin (also increases GI motility) |
|
|
What are two viruses that break the rules of double stranded, icosahedralness?
|
1. Parvoviridae -- so simple it only has a single strand of DNA
2. Poxviridae -- large and complex, does not have icosahedral symmetry AND replicates in the cytoplasm as opposed to the nucleus. |
|
|
What macrolide antibiotic is safe in pregnancy? What adverse effect is caused by erythromycin given to infants less than 6 weeks of age for pertussis?
|
azithromycin
hypertrophic pyloric stenosis |
|
|
What are components of an enveloped virus?
|
1. Lipoprotein membrane
2. VAP -- used as antigen, used in creating vaccines 3. tegument layer -- matrix proteins, linking internal nucleocapsid assembly to envelope, usually not glycosylated |
|
|
What is the mech of action of tetracycline antibiotics? What are they used for?
|
binds to 30s subunit, inhibit attachment of aminoacyl-tRNA
fecally eliminated, can be used in patients with renal failure -- must not take with milk or iron. used for Lyme, ulcer, M.pneumo; accumulates intracellularly, so great for rickettsia and chlamydia |
|
|
List DS DNA viruses.
|
Herpes
Adeno Papilloma Pox Irirdio |
|
|
What is demeclocylcine used for?
|
SIADH via inhibiton of ADH receptors in renal collecting ducts
|
|
|
List +ssDNA virsus.
|
Parvo
|
|
|
How does choramphenicol work?
|
binds to 50s, inhibits peptidyltransferase
|
|
|
List DS RNA viruses
|
Reo
|
|
|
What are the adverse effects of chloramphenicol? What is chloramphenical primarily used for?
|
gray baby
aplasic anemia p450 inhib MENINGITIS (haem, neisseria, step pneum) |
|
|
List +ssRNA viruses
|
Flav
Hepe Picorna Corona Toga |
|
|
What are the mech and side effects of linezolid
|
binds 50s
inhib MAO, lactic acidosis, peripheral neuropahy, optic neuritis |
|
|
List -ssRNA viruses
|
Filo
Paramyxo Orthomyxo Rhabdo Arena |
|
|
Why is imipenem given with cilstatin?
|
cilstatin is inhib of renal dihydropeptidase, which ordinarily would inactivate imipenem in renal tubules
|
|
|
List +ssDNA w/DNA intermediate
|
Retro
|
|
|
Which gram neg rods with ampicillin kill?
|
Haem
E coli Listeria Proteus Salmonella enterococi |
|
|
DS w/RNA intermediate
|
Hepadna
|
|
|
Which drugs kill pseudomonas?
|
car, tic, piperacillin
imipenem/cilstatin aztreonam fep, fop, taz fluoroquinolones aminoglycosides |
|
|
Describe Attachment of a virus in the early phase of viral replication.
|
i.surface proteins to cellular factors
1.naked viruses recognized cell receptors 2.enveloped viruses viral proteins embedded into the envelope ii.temperature independent step – iii.cell surface receptors 1.species specific and tissue tropism 2.cells w/o appropriate receptors are not susceptible to the virus 3.some viruses require a second protein besides the initial receptor for added specificity 4.receptors usually has other properties and functions in that cell a.ie. CD4 receptor used by HIV iv.Viral attachment proteins (VAP): 1.lipoprotein on the virus 2.some have more then one VAP (pox, herpes) 3.may have several domains that act with different receptors v.binding of VAP to receptors sometimes aids in the viruses entry into the cell |
|
|
What are the toxicities of polymixins?
|
neurotoxicity, nephrotoxicity
|
|
|
Describe Penetration of a virus in the early phase of viral replication.
|
i.energy DEPENDENT step
ii.depends of viral structure and cell type iii.penetration differ 1.naked viruses capsid protein rearrangement allows penetration 2.enveloped – direct membrane fusion a.pH independent process b.ie. HIV iv.both types can enter via endocytosis or fusion of virion envelope (enveloped viruses only) with host membrane 1.pH dependent process – requires Acidic endosome in order for the membrane to fuse with that of the endosome (uncoating requires low pH) 2.fusion of viral membrane with endosome a.“free ride” deep into the cytoplasm 3.triggers and promotes uncoating of viral proteins 4.leaves nor viral glycoproteins on cell surface no triggering of immune system |
|
|
What are the mech of the TB drugs?
|
1. INH -- inhibits mycolic acid synth
2. rifampin -- RNA polymerase inhibivtor 3. pyrazinamide (unknown) 4. ethambutol -- inhibits RNA synthesis |
|
|
Describe Uncoating of a virus in the early phase of viral replication.
|
i.release of viral genome from the outer structural components of the virion
ii.viral genome released: 1.as free nucleic acid 2.nucleocapside iii.DNA viruses that need to get to the nucleus are taken to nuclear pores and contents let out there iv.naked virus endocytosis and release from endosome 1.interaction of viral particle with endosome membrane causes vesicle formation a.virus genome just pours out of the endosome 2.virus capsid goes to the nucleus pore-like structure in the membrane – with no lysis a.just go right to pore and release genome into the nucleus v.enveloped- fusion of virus envelope with endocytotic vessel 1.can either lyse the endosome membrane vi.intracellular transport: 1.endocytotic vesicles 2.capsid protein interact with cellular motor proteins |
|
|
What are the adverse effects for TB drugs?
|
rifapin -- hepatotox, red orange, P450 inducer
INH -- SLE, seizures, hemolysis in G6PD, hepatitis, peripheral neuropathy (supplement with B6) Pyrazinmide -- increased porphyrin synthesis, hepat, hyperuricemia Ethambutol -- optic neurtitis, decreased visial acuity, hyperuricemia |
|
|
Describe Replication of a virus in the late phase of viral replication. (DNA)
|
i.DNA viruses (transcription and replication in the NUCLEUS):
1.need to make mRNA later on translated a.makes mRNA just like we do using splicing machinery in the nucleus b.then mRNA moves to cytoplasm where translation occurs 2.needs to replicate genome 3.needs to enter nucleus to use eukaryotic machinery a.use nuclear localizing signal- viral nuclear targeting (like ours do) b.nuclear pore complex disassembl and interact with importin i.capsid binds to cystosolic side of pore and DNA injected c.during mitosis viruses enter (retroviruses not HIV) ii.dsDNA virus enters nucleus 1.viral genome uses Host DNA dependent RNA polymerase to synthesize their mRNA a.select few viruses bring their own viral DNA polymerase (ie. adenovirus) b.Problem for virus: our DNA polyemerase and dNTP are only there when we are replication (overcome by): i.some replicate only in growing cells ii.some stimulate cell growth and DNA synthesis (papovirus) iii.some encode enzymes to provide dNTP and DNA synthesis (herpes) 2.viral mRNA exits nucleus to the cytoplasm where it is translated into viral proteins uses host ribosomes a.early proteins enzymes i.most viruses make virus encoded polymerase (replicase) that replicates the genome b.late proteins structural i.some need to be cleaved first by proteases other are made directly into structural proteins 3.naked genome is infectious |
|
|
How to treat VRE?
|
linezolid and streptogramins (quinupristin/dalfopristin) -- bind 50s
|
|
|
How does cytoplasmic DNA virus replicate?
|
i.poxvirus
1.brings its own DNA dependent DNA polymerase (replicase) and Virion DNA dependent RNA polymerase 2.has a very large genome in order to bring all these proteins 3.viral genome is NON-infectious a.take naked genome in cell- without the proper proteins it wont be able to replicate 4.initial transcription occurs in core of virion a.needs to code for cytoplasmic RNA and DNA polymerase b.needs to code accessory proteins needed for DNA and RNA syntheisis |
|
|
What are the side effects of AmpB?
|
fever/chills
hypotension nephrotoxicity arrhythmias anemia IV phlebitis reduce toxicit with hydration or liposomal Amp |
|
|
How does gapped DNA virus replicate?
|
i.Hepatitis B
1.have partially dsDNA virus 2.goes into nucleus first thing it has to do is repair missing piece of dsDNA using virion DNA polymerase 3.then uses the host RNA polymerase to create viral mRAN 4.virion has RNA dependent DNA polymerase produces via reverse transcription 5.naked genome is non-infectious a.b/c first step it to use virion DNA polymerase |
|
|
How does capsofungin work? What is it used to treat?
|
inhibits cell wall synthesis by inhib b-glucan
Invasive aspergillosis |
|
|
Describe Replication of a virus in the late phase of viral replication. (RNA)
|
i.RNA viruses that do not copy their RNA into DNA
1.need RNA dependent RNA polymerase 2.ALL RNA viruses encode this RNA dependent polymerase a.no viral proteins can be made until viral mRNA can be made 3.+ RNA virus a.+ strand serves as mRNA – create proteins directly from free ribosomes in our cells i.+ genome used to create RNA dependent RNA polymerase 1.this is then used to copy the + RNA viral genome to produce ss - RNA b.+ strand also serves as a template for synthesis of complementary – strand c.naked genome is infectious because can be immediately transcribed from the already existing + strand 4.- strand RNA viruses a.first negative has to to copy into an mRNA (+ strand) i.– strand not recognized by our ribosomes b.then the plus-sense (+) strand can be used to make proteins c.this naked genome is not infectious- because needs its own virion RNA dependent RNA polymerase to transform – into + and then proteins can be made |
|
|
How does terbafine work?
|
inhibits fungal enzyme squalene oxidase
|
|
|
Describe Replication of a virus in the late phase of viral replication. (retrovirus)
|
i.viruses which copy RNA into DNA
1.retrovirus ss diploid + RNA a.does not function as mRNA immediate do not undergo immediate translation 2.use virion RNA dependent DNA polymerase: transcribes + strand to – strand 3.– strand enters nucleus of host cell using host dependent DNA polymerase a.turned into dsDNA incorporated into host genome b.then uses host genome RNA polymerase to make mRNA proteins 4.naked genome is non-infectious |
|
|
Flucanazole is choice for?
|
cyrptococcal meningitis in AIDSs patients and candida
|
|
|
Describe Protein Synthesis of a virus in the late phase of viral replication.
|
i.mRNA goes to cytoplasm and ribsosomes translate it into protein
ii.our protein synthesizing machinery only translates monocistronic massages: 1.only produce 1 protein translated from an mRNA (starts at the 5’ cap and ends at a poly A 3’ tail) iii.strategies for viruses: 1.DNA virus uses our cell machinery a.genes are transcribed from both strands in opposite direction b.also can use the splicing machinery 2.RNA virus a.some viruses have to have on RNA 5’ methylated cap i.ones that don’t have 5’ cap have IRES – recognized by our cell ribosomes ii.some viruses bring poly A tail to stop translation b.some RNA viruses have polyprotein precursor that is later on cleaved by proteolytic proteins (host or viral) c.multiple monocistronic mRNA- multiple replicate small mRNA and from each produce 1 specific protein d.unusual example nuclear capsid transported to nucleus and mRNA synthesis made in nucleus i.uses our splicing machinery smaller mRNA then translated into many proteins |
|
|
Ketoconazole is choice for?
|
basto
coccio histo candida hypercortisolism |
|
|
Describe Assembly and Release of a virus in the late phase of viral replication.
|
i.process begins when concentration of the structural proteins and viral genome in cella re sufficient
ii.assembly site 1.DNA viruses – in the nucleus (except poxvirus) 2.RNA viruses – in cytoplasm iii.Naked virions: 1.protein forms protomers capsomeres procapsid a.procapsid- empty capsid that is not final or stable yet 2.genome enters procapsid 3.virion released by cell lysis or may be released by reverse phagocytosis iv.enveloped viruses: 1.newly synthesized and processed viral glycoproteins are delivered to cellular membrane by vesicular transport 2.viral proteins associate with viral nucleic acid to form nucleocapsid 3.envelopes are formed around nucleocapsid by BUDDING of cellular membrane a.budding as explained above sindbisvirus b.another way budding can occur where assembly of proteins occurs on the membrane itself i.host proteins are displaced ii.ie. influenza virus c.use plasma membrane: i.ie. alpa virus, arenavirus… 4.some viruses use the internal membrane as their outer membrane: a.cox virus golgi membrane b.herpes nuclear membrane v.Symmetry assembly 1.Isosahedral symmetry a.empty structures can be procaspid b.or are assembled around the genome c.is a chance that you can have a isahededral symmetry without genome 2.helical symmetry cannot form without viral genome |
|
|
What is contraindicated in those with AIP?
|
Griseofulvin
Barbituates Anticonvulsants |
|
|
Describe recombination in virus.
|
i.breakage reunion mechanism end get virus with gene that isn’t present in either parent
1.can happen when 2 different viruses are infecting the same cell ii.marker rescue- take wild-type DNA fragment and a lethal mutant 1.put together can rescue the mutant 2.used in the lab iii.RNA virus copy choice mechanism 1.2 different RNA viruses that are close polymerase starts to transcribe one viral genome and then jumps to the other viral genome a.end up with virus with 2 parental origins iv.genetic reassortment with segmented genome 1.more then one virus in the cell when the virus is assembled it mixes 2 genomes |
|
|
What is the mech for pyrimethamine?
|
selectively inhibits plasmodial DHFR. drug of choice for toxoplasmosis when combined with sulfadiazine
|
|
|
What are some nongenetic alterations during viral replication?
|
i.interaction of viral gene products in cell infected with 2 viruses
ii.complementation: 1.one virus has defect in capsid when it infects cell with another virus it can use the capsid of the other and gain back infectability iii.phenotypic mixing 1.2 different viruses infecting the same cell one uses the other’s proteins to produce another virus (pseudotype-different type of virus) |
|
|
What is the mech for suramin?
|
inhibits enzymes involved in eergy metabolism. No CNS involvement (like melarsoprol)
|
|
|
What types of viral infections exist?
|
1. Acute infection – virus enter body, reproduce, recovery.
2.Acute infection with rare late complications – After the disease, the virus is no longer in the body, but occasionally, viral capsids can be found. Can be detected every now and then. After several years, there can be a related disease episode. 3.Latent infection – Start with acute infection, then there is decline in disease and recovery; however, the virus has not actually disappeared from the body. It remains in certain tissues in dormant form. It can activate and form new viral particles when conditions are appropriate. 4.Chronic infection – Infection, then continual replication and shedding. Disease symptoms reoccur. 5.Chronic infection; late disease – when the virus enters the body, it does not cause disease initially. It remains and replicates; only when there is much accumulation, there will be a late presentation of the viral disease 6.Slow infection – virus stays in the body (not completely latent). Very slow replication, but the virus eventually accumulates. Late disease onset. |
|
|
What is the mech for nifurtimox?
|
forms free radicals
|
|
|
What are mycorrhizae?
|
They are fungus on and in the roots. Symbiotic relationship. Giving protection. There is greatly increased surface area of roots. The plant, in return give the fungi back carbs and sugars.
|
|
|
What is the mech of sodium stibogluconate?
|
inhibits glycolysis at PFK reaction
|
|
|
How does penicillin kill?
|
It inhibits transpeptidase, which cross links PDG cell wall of gram positive bacteria.
|
|
|
What is the mech for chloroquine?
|
blocks plasmodium heme polymerase, leading to accumulation of toxic hemoglobin breakdown products that destroy organism
|
|
|
How are the classifications of fungi divided?
|
1. Zygomycota -- reproduce sexually by forming a zygospore (most primitive fungi, seen on food)
2. Ascomycota -- largest family (medical fungi), forms sexual bodies called ascis (little sacs) 3. Basidomycota -- make mushrooms, basidia fruiting bodies create spores (result of sexual interaction w/in mushroom cap) |
|
|
What is mech for mabendazole?
|
inhibits glucose uptake and MT synthesis?
|
|
|
What are defining fungal characterisitics?
|
1. Eukaryotes w/cell wall
2. Immobile 3. Heterotrophic (animal eating) 4. Uni/Multiceullular (same cell type) 5. Spore formation |
|
|
What is mech for pyrantel pamoate?
|
stimulates nicotinic receptors at NMJ. contraction occirs, followed by depolarizing-induced paralysis. No effect on tapeworms or flukes.
|
|
|
What two types of fungal morphology exist?
|
1. Yeast -- round, egg shaped. Spend less energy on cell wall, mobile, reproduce by budding & fission
2. Mold -- wasted energy by leaving behind tails of dead mycinium, good for pathogenesis (great for digging) |
|
|
What is the mech for ivermectin?
|
intensifies GABA-mediated neurotransmission and causes immobilization. Does not cross BBB
|
|
|
What types of yeast classifications exist?
|
1. Budding (round)
2. Fission (Elongated) 3. Pseudohyphae (chains of cells that bud from one another -- good for digging) |
|
|
What is the mech for praziquantel?
|
increases membrane permability to Ca, causing contraction and paralysis of tapeworms and flukes.
|
|
|
What hyphal types exist?
|
1. Septate -- have cross walls; if one area is damage, it seals off; presents structural support
Worronin bodies will rush to plug up the hole b/w the speptate if a segment is damaged. 2. Aseptate -- no cross walls; grow very quickly |
|
|
What is ribavirin mech? What is its clinical use Toxicities?
|
mech -- inhibits synthesis of guanine nucleotides by competiviely inhib IMP dehydrogenase.
use -- RSV, chronic hep C, arenavrisu tox -- hemolytic anemia, severe tetratogen |
|
|
What would happen if we removed the cell wall from a live fungus?
|
It would burst and explode because of osmotic pressure.
|
|
|
What is the mech for foscarnet? Use? Tox?
|
viral DNA polymerase inhib that binds to pyrophosphate-binding site of enzyme. Does not require thymidine kinase activation.
Used for CMV retinitis (gangciclovir too) Hematotoxicity, nephrotoxicity |
|
|
What is in the fungal cell membrane?
|
Ergosterol (like cholesterol)
Enzymes Receptors Channels Spits out cell wall components |
|
|
What are the toxicities of gangcylovir?
|
leukopenia, neutropenia, thrombocytopenia, renal toxicity
|
|
|
What are the parts of the fungal cell wall?
|
1. Inner -- chitin (N-acetyl glucosamine linked) and glucans 1-3 and 1-6
2. Outer -- mannoproteins: proteins w/mannose residues connected to glucans in inner layers. Our immune system senses mannoproteins. |
|
|
What is the life cycle of a typical mold (aspergillus fumigatus)?
|
1. Conidium (spore) swells and germinates, take up
2. Forms a hyphae, nuclcei divide 3. 24 hrs --> form highly branched mycelium 4. Some will grow up into the air (aerial) 5. These will form conidiophores (structures that will asexual make spores) |
|
|
What is the typical life cycle of a typical yeast (saccharomyces cerevisiae)?
|
1. Swelling then bud formation
2. Nucleus moves b/w the divisions 3. The two cells will separate Leaves a scar on mother |
|
|
What are asexual mold spores?
|
1. Arthroconidia -- Most primitive way of making spores: parts of mycilium thicken and detach.
2. Blastic Conidiogenesis -- spitting out chains of spores. |
|
|
What are some spore dispersal strategies?
|
1. Underside of mushroom contains hundreds of leaflets containing spores
2. Omphaletus -- glow in the dark! attracks moths which will come and disperse 3. Cup -- explosive! 4. Pilobolus -- when touched, shoots out. very sticky e.g. sticks to grass. Cow will eat it and poop it out. Cycle starts over. |
|
|
Describe Picornavirus. What are examples?
|
+ssRNA (genome & mRNA same)
1. Enteroviruses Polio Coxackie A, B Echo Entero Encephalomyelitus Encephalomyocarditus 2. Rhinovirus Foot and mouth 3. Cardiovirus |
|
|
What is the mech of protease inhibitors? Tox?
|
prevent maturation of new viruses by inhibiting HIV-1 protease, which starts assembly by cleaving polypep productics of HIV mRNA into functional counterparts
-- navir tox -- hyperglycemia, GI intolderance, lipodystrophy, thrombocytopenia |
|
|
What are the host cell receptors for the following?
1. Hu Rhino 2. Polio 3. Caxsackie A 4. Echovirus 5. EMCV |
1. ICAM-1/LDLR
2. CD155 3. ICAM-1 4. VLA-2/DAF 5. VCAM-1 |
|
|
What are some disease associations with picorna? Why is this important?
|
1. Paralytic Disease (polio, entero71)
2. Meningitis (all entero) 3. Encephalitis 4. Fever (all entero) 5. Hand/foot/mouth disease (Cox A) 6. Herpangina (Cox A) 7. Bornholm Disease (Cox B) 8. Respiratory Infection 9. Rubelliform rashes (cox A, B, echo) 10. Conjunctivitis (cox A24, entero70) 11. Pancreatitis/Diabetes (cox B) 12. Neonatal Infection (cox B, echo) 13. Myocarditis (cox B) Many cause similar responses. Need differential diagnosis. |
|
|
Describe the pathogenesis of the poliovirus.
|
1. Virus enters peyer's patches and tonsils (better replication). Has both fecal-oral and oral-oral transmission
2. Travels to deep cervical lymph and mesenteric lymph --> transient viremia 3. Viremic phase 4. Neurological phase: moves to regional nerve ganglia to CNS, crossing blood brain barrier |
|
|
Describe nucleotide substitutions in polio.
|
1. Most observed substitutions are synonymous; most are in 3rd codon position.
2. Some substitutions change the phenotype (neutral, good, bad) |
|
|
How can efficacy of polio vaccination be established? Why?
What types of vaccines exist? |
Need serosurvey. Most cases are asymptomatic.
1. Inactivated IPV (stable) 2. Attenuated OPV (unstable) |
|
|
What types of polio can be seen in the environment?
|
1. Vaccine (OPV)
2. Vaccine derived (evolved from OPv0 3. Wild |
|
|
What is the "magic number" for herd immunity?
|
95%
|
|
|
Why does polio vaccination fail?
|
1. High birth rate
2. High NPEV infection rate -- interference 3. High force for transmission 4. AFP in children receiving > 10 OPV doses |
|
|
What are vaccine derived polio?
|
Evolved from vaccine.
Genetic divergence is b/w 1% and 15%. Arise by: 1. Circulation (cVDPV) from person to person 2. Persistent infection of immune deficient individual (iVDPV) 3. Unknown/ambiguous (aVDPV) |
|
|
Describe aVDPVs.
|
1. Neovirulent in transgenic mouse model
2. Many changes in neutralizing antigenic sites. 3. Persistent intermittent detectable excretion over long periods of time. |
|
|
What is gastroenteritis?
|
Syndrome with GI symptoms (nausea, vomiting, diarrhea, abdominal discomfort)
|
|
|
What viruses are responsible for acute gastroenteritis?
|
1. Rota (6-24 months)
2. Enteric adeno (<2 years) 3. Astro (<2 years) 4. Noro (<2 years) |
|
|
On EM, how do the following viruses look (in increasing size)?
1. Entero 2. Norwalk 3. Astro 4. Calici 5. Rota 6. Adeno |
1. Spherical; smooth edge, little surface detail
2. Spherical; smooth edge, little surface detail 3. Spherical; 5 or 6 pointed translucent star with triangular hollows in 10-20% of particles 4. Spherical; 6 peripheral hollows around one central hollow 5. Spherical; double capsid, capsomers radiate out to smooth rim 6. Hexagonal; tightly packed distinct capsomers |
|
|
What are the genomic structures of the following RNA viruses?
1. Noro 2. Astro 3. Picorna 4. Rota |
1. +SS
2. +SS 3. +SS, polyprotein 4. DS, 11 segments |
|
|
In rota, what is the difference b/w VP7 and VP4?
|
VP7 -- characterized by neutralization, 9 of 14 found in humans
VP4 -- not enough antisera to characterize, characterized by sequence analysis, 9 of 20 found in humans |
|
|
What are the stages in rota replication?
|
1. Absoption, Penetration, and Uncoating
- glycocongugate receptor binding - receptor mediated endocytosis or direct penetration - entry into cytoplasm 2. Transcription and regulation - viral enzymes RNA polymerase - + and - RNA strand synthesis - protein synthesis 3. Assembly - 1 and 2 shell virion - bud through ER (temporary envelope) - new protein coat 4. Release - via host cell lysis |
|
|
What is the time course of infection for Noro?
|
There is absence of bacterial or parasitic pathogens. Vomiting > 50% of cases; duration 12-60 hr; incubation 24-48 hr
|
|
|
What immune cell types exist?
|
1. Myeloid cells --
Granulocytes: Neutrophils, Basophils, Eosinophils Monocytes: Macrophages, Kupffer Cells, Dendritic Cells 2. Lymphoid Cells -- T Cells: Helper, Suppressor, Cytotoxic B cells: plasma cells Natural Killer |
|
|
What are the four types of defensive barriers in innate immunity?
|
1. Anatomic (Skin & sebum, gut villi)
2. Physiologic (temperature, pH, soluble factors: lysosyme, collectin, interferons, complement proteins) 3. Phagocytic (macrophages, dendritic cells, monocytes) 4. Inflammatory (basophils, mast cells) |
|
|
What are mononuclear phagocytes?
|
Monocytes, macrophages, dendritic cells. Involved in phagocytosis and killing of microbial pathogrens, elimination of apoptotic cells, tissue repair/would healing, antigen processing and presentation and regulation of innate and adaptive immunity by cytokines and chemokines.
|
|
|
What are polymorphonuclear phagocytes?
|
Include neutrophils (major white blood cell). Phagocytoze and kill microbial pathogens. Originiate from bone marrow where there is resevoir and release when needed to fight infection. Live 6-8 hours after release and live under high stress in circulation.
If there is a change greater than 8% in neutrophils in bone marrow, body is fighting infection. |
|
|
What are the three major events that occur during an inflammatory response?
|
1. Vasodilation
2. Increase in capillary permeability (development of exudate) 3. Influx of phagocytes (Margination -- phagocytes adhere to wall of blood vessels, Diapedsis -- immigration to tissue, Chemotaxis -- migration to site of invasion) 4. Mediation |
|
|
Which cells express CD 4 function? CD 8?
|
T helper. T cytotoxic.
|
|
|
What do natural killer cells do?
|
Stop production of the virus. Lymphocyte-like cells that lack antigen-specific receptors. They can detect and attack certain virus-infected and tumor cells. Identified by the presence of CD 56, CD16, and absence of CD3. Activated by IL2 and IFN-gamma to become LAK cells.
|
|
|
What are PAMPs?
|
Large groups of molecular patterns that are specific to pathogens in general, such as composition of cell wall, presence of viral nucleic acids.
|
|
|
What are the two types of phagocytosis?
|
1. Non-opsonic -- direct recognition of bacteria such as mannose receptor
2. Opsonic -- dependent on opsonins, which are elements that coat the bacteria and act as a bridge b/w the bacteria and the phagocyte surface receptors (complement derived factors, C-type lectins, specific antibodies) |
|
|
Which type of infection is more prevalent in patients having macrophages with impaired killing activity?
a)Viruses b)Extracellular bacteria c)Intracellular bacteria d)All of the above |
Macrophages have some tole in antiviral immune response, but it is not the only or the MAIN mechanism. Extracellular bacteria have several different components that can deal with it (neutrophils, etc). Intraceullar are problematic b/c they invade macrophages and have anti-macrophage mechanisms. They tend to avoid elimination, and a classical example is Smiley salmonella <3. The answer is C, intracellular bacteria.
|
|
|
What is the oxidative burst?
|
Rapid realease of reactive oxygen species from different types of cells. Denotes the release of chemicals from immune cells as they come into contact with bacteria. It is a crucial reaction that occues in phagocytes to degrade internatlized particles and bacteria. NADPA oxidase produces superoxide, which spontaneously recombines with other molecules to produce free radicals. The superozide reacts with NO, which will redeuce bioactive NO. To combat infections, immune cells use NADPH oxidase to reduce O2 to fogygen free radical. Neutrophils and monocytes utilize myeloperoxidase to further combine H2O2 with Cl+ to produce hypochlorite, which will help destroy bacteria.
|
|
|
What is the mechanism for the oxidative burst?
|
1. NADPH oxidase
2. Generates superoxide 3. Superoxide dismutase 4. Superoxide converted to H2O2 5. Myeloperoxidase + Fe 6. Peroxide converted to OCl and OH 7. Direct bactericidal effect |
|
|
What are some hereditary defends in phagocyte respiratory burst?
|
1. Myeloperoxidase-independent: NADPH oxidase deficiency -- Chroinc Granulomatous Disease; severe illness, recurrent severe bacterial infections, death in early life
2. Myeloperoxidase-dependent: MPO deficiency -- less severe illness, recurrent bacterial infections, survival! |
|
|
What are the roles of dendritic cells?
|
antigen collection from the periphery, regulation of immune response, professional antigen presentation, and activation of adaptive immunity. Its functions include pahgocytosis & macropintocytosis, secretion of cytokines and chemokines, antigen processing, and migration to lymph nodes
|
|
|
What are the steps in dendritic cell activation?
|
1. DC migration via afferent lymphatics -- antigen from sites of infection reach lymph node
2. Presentation and activation in draining LN 3. Activated T cells travel from the draining LN to the inflamed tissue 4. Antigen specific T cells recruit macrophages to facilitate inflammation |
|
|
Patients treated with intense chemotherapy are usually exposed to the immediate threat of:
a)Bacteria b)RNA viruses c)DNA viruses d)Parasites |
Chemotherapy does not kill bacteria. It eliminates cells that proliferate rapidly, such as epithelium cells. Neutrophils tend to be the most rapidly proliferating cells, so these will be the ones that are affected first. Neutrophils swallow bacteria and fungi.
|
|
|
How does the mannose receptor work>
|
Within each of the clusters, the carbo-binding sites have a fixed orientation.
MBL binds with high affinity to mannose and fructose residues with correct spacing. MBL binds to mannose recptor C-type lectin. Mannose and fructose residues that have different spacing are not bound by MBL. Discrimination from "self" through carb spacing pattern. |
|
|
What cell surface receptors exist for opsonins?
|
1.Fc receptors (for antibodies)
2.Complement receptors 3.Collectins (MBL, surfactant proteins A & D) Connection b/w elements of immune system; generally, enhance phagocytosis |
|
|
What are NOD proteins?
|
Identify bacteria, so binding domain similar to that of TLRs. ABle to recruit proteases.
NOD1 expressed in epithelium. NOD2 in cytosol can detect bacterial proteoglycans. Leads to activation of transcription factor NFxB and expression of pro-inflammatory genes. |
|
|
What does RIG I do?
|
It is involved in double stranded domain recognition and activates type I interferon.
|
|
|
What are the unique features of Herpes?
|
1. large, iscohedral, enveloped linear DS DNA virus
2. Encodes DNA plymerase 3. Ubiquitious; cell-mediated immunity required for control |
|
|
What are the three subfamilies of Herpes? How are they characterized?
|
Based in vivral characterisitcs and pathogenesis.
1. α = HSV 1 + 2 and varicella zoster virus a) all infect epithelial cells primarily b) cause latenet infection in neurons 2. β = cytomegalo virus and human herpes virus 6 a) infect and become latent in variety of tissue 3. γ = EBV and Human herpes virus 8 a) infect and become latent in lymphoid cells b) many associated with malignant diseases |
|
|
NF2
|
22q;
NF type 2 [bilateral acoustic neuroma, juvenile cataracts] (tumor suppressor) |
|
|
What are the virokines of herpes simplex? What do they do?
|
1. gE-gI = binds Fc of IgG, preventing complement-mediated cytolysis
2. C-1 = Binds C3b, inhibiting alternative and classical pathways |
|
|
What is the mechanism of spread of herpes.
|
1. Virus enters mucosal tissue, forming a local infection with local spread. It will replicate in epithelial cells.
2. It will pass to neighboring cells. 3. Induces Cd4 mediated responses, cytotoxic cells, NK cells. 4. Causes formation of lesion, ulcerization, then healing. 5. Antibodies are made to stop the virus from spreading. Most virus will be destroyed; however, some manage to escape and bind to endings of sensory neurons. 6. Climb through retrograde transport in dorsal root ganglion and reach body. This is aided by cellular proteins dynein and dynactin with tegument proteins. 7. Virus will remain dormant in the neuron until there is stimulus for reactivation (stress, UV, light, fever, etc -- causing local immune suppression) 8. Anterograde transport facillitated by kinesin along sensory nerve, reaching epithelium and starting all over again.1 |
|
|
What is the advantage of Herpes staying in the neuron?
|
1. Express no MHC antigens, shielded from lysis by cytotoxic T lymphocytes. B/c latent viral genome express no protein, also safe from lysis by antibody plus complement or ADCC.
2. Neurons do not divide, the virus has no need to divide to maintain fixed number. 3. axon provides a direct pathway to periphery, to susceptible epidermal cells |
|
|
Describe Acute Gingivostomatitis
|
Self limiting disease (13 days)
Pain and bleeding of gums |
|
|
Describe Herpes Labialis
|
Cold sore.
|
|
|
Describe Ocular Herpes
|
Remains to trigeminal nerve.
Primary HSV keratitis, dendritic ulcers Conjuctivitis Cornea damage |
|
|
What is the one of the most serious complications of herpes simplex?
|
Encephalitis.
Neonatal -- global involvement, brain is almost liquefied. Mortality rate approaches 100% Focal disease (children, adults) -- temporal lobe most commonly affected. Mortality w/out treatment 70% |
|
|
What is given to adults and neonates for herpes simplex?
|
Acyclovir
|
|
|
What other parts of the body can herpes simplex infect?
|
Liver
Spleen Lungs CNS |
|
|
How to diagnose HSV infections?
|
1. Direct microscopic examination -- see mutlinucleated cells, type A inclusion bodies
2. Cell culture -- see replication and CPE 3. Assay |
|
|
What is so amazing about acyclovir?
|
1. Acyclic
2. Once it enters enlongating DNA, stops sugar moities. 3. Drug has to undergo 3 phosphorylations: a) first by virus (can only be actived by infected) b) second, cellular enzymes (will be incorporated with viral DNA) Less side effects; however there is chance for resistance. |
|
|
What are the properties of Zoster and Varicella?
|
1. belongs to alpha subfamily of herpes
2. DS DNA 3. One antigenic serotype only, though there is some cross reaction with HSV 4. Replicates slower than HSV, in fewer types of cells |
|
|
How does VZV differ from HSV?
|
There is no asymptomatic shedding.
|
|
|
What is the mechanism of spread of VZV in body?
|
1. Droplets to respiratory tract to lymphatics to liver, spleen, reticuloendothelial system
2. Viremia (fever, malaise, headache, sore throat) 3. Eruption in skin (macules to papules to vesicles to pustules to crusts) 4. Latency in neuron |
|
|
How can VZV be diagnosed?
|
1. Cytology -- Cowdry's Type A inclusion bodies
2. Antigen detection 3. Virus isolation 4. Serology -- Increased IgM and Ab are increased |
|
|
What is Varivax?
|
Live vaccine for VZV
|
|
|
What are properties of the cytomegalovirus?
|
1. Belongs to betaherpes
2. DS DNA 3. Largest genome 4. contains RNA mRNAs w/in virion |
|
|
Why is CMV so successful as a human pathogen?
|
1. Transmitted vertically or horizontally with little effect on the host
2. Carried for life with reactivation |
|
|
Where does CMV establish latency?
|
T cells
Macrophages Stomal cells of bone marrow Kidney epithelial cells |
|
|
What are the clinical manifestations of CMV?
|
1. Congential -- cytomegalic inclusion disease. Usually asymptomatic except in a few neonates who get fever, lyphadenopathy, spelnomagaly
2. Immunocompromised -- (like in people with AIDS) pneumonitis, retinitis, colitis, encephalopathy |
|
|
What is an epitope? How does the body react to it?
|
A site on an antigen that is recognized by an antibody. Every epitope causes the body to generate an antibody against that epitope. An antibody can recognize ONE epitope. One plasma cell produces antibodies for ONE eptiope (clone)
|
|
|
What is the general structure of an antibody?
|
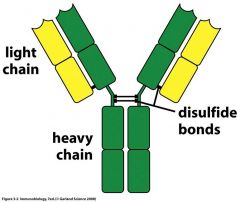
|
|
|
In an antibody, what types of light chains are there? What type of heavy chains? What is special about this?
|
Light -- lamba, kappa (2x more lamba in humans)
Heavy -- a,g,d,e,m This affects class and effector function. |
|
|
Describe key elements about IgG. What is its mechanism?
|
It has 4 different isotyes. (IgG1-4); IgG3 has the shortest half life (8 days). They all differ in their disulfide bonds. A mother can deliver these all to the uterus. Comprise 80% of all immunoglobulins.
IgG molecules bind to antigens on the bactgerial surface. Cq1 binds to at least two IgG molecules. |
|
|
What are the characteristic of IgM moecules? Where are they produced? What form is most useful?
|
These are the first Ig class produced in primary response to an antigen. It is also the first synthed by neonate. Membrane IgM expressed by immature and mature B cells. 5-10% of serum Ig.
Pentomeric form is more useful in fighting pathogens. It has 5 Fc portions, which are efficient in complement activation (complement system is the one of the most important in getting rid of antigens). 10 Fab portions, which gives high avidity, blocking, and neutralization. Pentameric Igm molecules bind to antigens on the bacterial surface and adopt a “staple” form (like landing on the moon). A C1q molecule binds to one bound IgM molecule. |
|
|
What are the biological functions and characteristics of IgA?
|
1. Hallmark component of mucosal immunity
2. 10-15% of serum immunoglobulins 3. Found in breast milk and in mucous secretions of GI, GU, bronchial, tears, saliva 4. Prevents pathogen binding and colonization 5. Facilitates pathogen entrapment in mucous 6. Passive protection to the newborn 7. Secretion of IgA is greatest than all other Ig 8. 5-15 g are secreted daily |
|
|
What is transcytoses?
|
Active transport of antibodies through epithelial barrier. Main function of IgA and IgM.
Mediated through: poly Ig receptor, J-chain, secretory component. In the lumen, poly Ig rector has a stem that will bind to IgA. The ply Ig receptor can sometimes bind directly to the bacteria. |
|
|
What is IgE involved with?
|
Allergic reactions
|
|
|
Describe IgE function.
|
1. Bind Fc receptors on the membrane
2. Fab binds epitopes on antigens generally called Allergens 3. Cross linking (activation) results in mast cell/basophil degranulation. |
|
|
What is the positive feedback loop of IgE response?
|
IgE secreted by B cell binds to higf-affinity Fc recptor FcR1. When crosslinked, secretes histamines and IL 4, which generates more IgE. Mast cells upregulate CD40 ligand. B cell can interact with CD 40, and the mast cell has CD40L. Activated mast cells provide contact and secreted signals to B cells to stimulate IgE production.
|
|
|
What is the variable region in an antibody? The constant region?
|
1. Variable -- specific binding to molecules from pathogens that elicit the immune response
2. Constant -- recruitment of other cells/molecules that will destroy the pathogen once the antibody is bound to it |
|
|
Describe variable region domains.
|
Variable region is composed of VH and VL
Substantial biochemical difference between antibodies Responsible for epitope binding Variability is mainly in 6 hypervariable sub-domains The H chain often contributes more to antigen binding |
|
|
What is avidity?
|
The overall strength of binding b/w an antigen awith many determinants and multivalent Abs. Non linear measurement
|
|
|
What is cross reactivity?
|
The ability of an individual binding site to react with more than one epitope
Cross reactions arise because: The cross reacting antigen shares an epitope with in common with the immunizing antigen Structral similar epitope to one of the immunizing antigens |
|
|
What are the clinical features of mumps?
|
1. Incubation 14-21 days
2. Replication in nasal mucosal and epithelial of URT 3. Penetration of draining lymph nodes (grows in activated T lymphocytes) 4. Transient viremia 5. Spread of infection to salivary glands, kidneys, pancreas, testes, mammary glands, CNS 6. Parotitis (bilateral) with fever. Virus damages tissue. Inflammation causes typical swelling -ssRNA virus |
|
|
Describe viral replication in mumps.
|
Sialoglyco-conjugate * HN
Endocytosis Fusion Transcription Translation & modification Replication Encapsidation Budding |
|
|
What is the immune response to mumps?
|
1. Humeral -- IgM, IgG, IgA; well established by 11 days post infection
2. Cellular -- cytotoxic T |
|
|
How to treat mumps and how to diagnose? What is a problem with vaccination?
|
1. Live attenuated virus vaccine or inactivated virus vaccine.
2. Viral isolation, serology (4 fold increase in IgG, IgM), antigen detection, RT-PCR Vaccination below eradication levels can mean naive individual may get infected later in life. Reduced incidence of infection, increased incidence of serious disease. |
|
|
Go through the epidemiology of measles.
|
Transmission is by airborne droplets
Highly contagious (R = 16-450) Nosocomial spread Virus shedding Starts during prodrome Can persist for 6 days after rash begins Maternal-derived immunity lasts 6-9 months Peak age of infection: 4 years |
|
|
What is measles?
|
-ssRNA
7-13 day incubation period Local replication in the respiratory tract Spread to lymphatic system Cell associated viremia Multi-organ dissemination Conjnnctiva respiratory tract urinary tract small blood vessels lymphatic system CNS |
|
|
What are the clincal manifestations of measles?
|
Beginning
Nasal congestion, eye redness, photophobia , cough and fever Looks sick Decreased activity and appetite Day 2 Koplik’s spots Day 3-4 Development of brown rash on face Spreads down the body Lasts > 3 days |
|
|
What are complications of measles?
|
Croup, bronchiolitis, and measles pneumonia
Bacterial super infection Pneumonia - 60% of measles related deaths Otitis media Postinfectious Encephalitis Fatal 15% of cases Immunopathological reaction causing demyelination of neurons |
|
|
How to diagnose measles?
|
Viral Isolation from urine
also Respiratory tract, Blood CSF, Brain Nucleic acid isolation directly from clinical samples RealtTime RT-PCR Sequencing & Molecular epidemiology Histology Multinuclear giant cells with cytoplasmic inclusion bodies (urine, URT) Serology IgM during rash Sero conversion or 4x increase in IgG |
|
|
Atypical manifestation of measles?
|
Prior sensitization with insufficient protection
More intense rash especially in distal regions Possible vesicles, petechiae, purpura or urticaria T-cell deficient children Bacterial infection Giant cell pneumonia without rash <25% mortality |
|
|
Chronic manifestation of measles?
|
SSPE Subacute sclerosing panencephalitis
Relatively rare (7 per million cases) CNS manifestation of persistent defective virus High levels of measles antibody in CSF Behavioral changes Personality memory changes myoclonic jerks spasticity Blindness Fatal coma within 1-2 yrs. |
|
|
How to prevent measles?
|
1. Vaccination
At least two doses live attenuated virus 12-15 months 4-6 or 11-12- yrs Usually together with Mumps and Rubella (MMR) Inactivated virus not effective Increased risks for atypical infections. Passive - immune globulin within 6 days post exposure |
|
|
Describe ruebella.
|
+ssRNA.
3 non-translated regions 2 ORFS post-translation cleavage |
|
|
How is the nucleic acid for rubella replicated?
|
1. Infecting genomic RNA
9757nt (+) ~ mRNA can serve as template for translation NS proteins necessary for replication 2. Transcription of subgenomic RNA 3327 nt (+) 3’ ORF for 3 structural genes C (core) E2 and E1 Capped and methylated 3. Transcription of Replicative intermediate 9757 nt (-) 4. Transcription of progeny genomic RNA 9757 nt (+) |
|
|
What is the infectious cycle of rubella?
|
Adsorption
viral GP with host cell receptors Penetration - fusion - release Receptor-mediated endocytosis Transcription - 3 RNA types (+) genomic (+) sub-genomic (-) Replicative intermediate Translation and post translational modification Cleavage of polyproteins Glycosylation Encapsulation Maturation - Budding Plasma membrane Vesicle membranes (avoid detection by immune system) |
|
|
What are clinical feature of rubella?
|
Early childhood
Often clinically asymptomatic Usually Mild (symptomatic see next slides) Adult More severe (joint pain) (See next slides) Gestational Abortion, miscarriages, still-birth, fetal malformation First 2 months - most fetuses infected Damage if fetus infected before 16th week (Symptoms in next slides) |
|
|
What is Brucella? What are its major pathogens and animal resevoir?
|
It causes Brucellosis
It is a gram neg rod without capsule i. Brucella Melitensis – goat and sheep ii. brucella abortus – cattle iii. brucella suis- pigs iv. brucella canis- dogs |
|
|
How is Cb3 used with CR1?
|
1. Bacertium coated with complement and IgG antibody
2. When C3b binds to CR1 and antibody binds to Fc receptor, bacteria are phagocytosed 3. Macrophage membranes fuse, creating a membrane-enclosed vesicle, the phagosome and eventually will be degraded |
|
|
How does antibody dependent cell-mediated cytotoxicity work?
|
1. Antibody binds antigens on the surface of target cells
2. Fc receptors on NK cell recognize bound antibody 3. Cross-linking of Fc receptors signals the NK cell to kill the target cell 4. Target cell dies by apoptosis |
|
|
What are the functions of the Fc receptor?
|
1. Enable cell-mediated effector functions of antibodes: phagocytosis (opsonization), sytotoxicity (ADCC)
2. Immunoregulatory signals by Ag-mediated crosslinking of FcR-bound antibodies: allergic reaction (degranulation of basophils, mast), enhancement/inhibtion of cell response (ITAM/ITIM) 3. Movement of AB across cell membrane 4. Transfer of IgG from mother to fetus across the placenta 5. Allow passive gaining of AB by many cell types |
|
|
What is IVIG?
|
Polyclonal Ab: passive immunotherapy.
Administration of pooled IgG from plasma (from many patients) Used for treatment for: 1. Immune deficiencies 2. Inflammatory and autoimmune diseases 3. Acute infections |
|
|
What is the mechanism of action for IVIG?
|
1. Modulation of complement activity
2. Suppression of autoantibody production 3. Saturation or blocking of signaling Fc recptors on macrophage cells and B lymphocytes 4. Supression of inflammaotry chemicals, such as the cytokines, chemokines, metalloproteinases 5. Blocking the Fc signal receptors 6. Autoantibody and toxin neutralization |
|
|
What are complement mediated functions?
|
1. Lysis
2. Opsonization 3. Activation of inflammatory response 4. Clearance of immune complexes |
|
|
Describe the pathways in which the complement system can be actived.
|
1. Classical pathway -- Ab binds to specific antigen on pathogen surface.
2. Lectin -- binding of proteins which have affinity for mannose. Bind to bacteria and other microbial systems. 3. Alternative -- Pathogen surface creates local environment conducive to complement activation |
|
|
What is the order of complement activation?
|
1. Alternative pathway if bacteria have C' activating surface molecules
2. Lectin pathway after production of mannose binding lectin (Mbl) by liver cells triggered by IL-6 (produced by activated macrophages) 3. Classical pathway after production of specific IgM and IgG antibodies. |
|
|
What types of fugal diseases are there?
|
1. Mycotoxicosis (ergotism)
2. Fungal Allergy 3. Mycoses |
|
|
What are fungal virulence factors?
|
Offensive -- attachment, toxins, immunomodulators
Defensive -- pigments, thermotolerance, capsule |
|
|
How do innate immune cells recognize fungi?
|
1. Carbohydrates in cell wall
2. Dectin-1 receptor 3. TLR 2,4 |
|
|
What are dermatophytes? How do they function? Where are they found?
|
They are ascomyetes, using amino acids and peptides to secrete keratinase.
Found in the soil, animals, and other humans. |
|
|
What fungus grows all over but never on the scalp? What fungus grows exclusively on the head?
|
1. T. Rubrum
2. T. Tonsurans, T. Violaceum |
|
|
Describe dermatophyte pathogenesis.
|
Two ways.
1. Direct contact: Adhesion to skin Spores germinate Proliferation Inflammatory Reponse (DTH around colony) -- ID response (papules all over body) 2. Contact with phomytes (most people have natural immunity) |
|
|
What are the different types of tinea pedis? What are the most common? How is it treated?
|
1. Interdigital
2. Plantar 'mocasin foot' 3. Acute plantar T. rubrum, mentagrophytes, E. floccosum Topical azole creams or systemic ones for systemic ones |
|
|
What is tinea ungium? What is the most common? How is it treated?
|
Invasion of nail plate. Progressive, persistent, infectious. Hard to treat!
T. rubrum. Long term systemic azole pills sometimes w/nail ablasion |
|
|
What types of tinea capitis are there?
|
1. Ectothrix -- "grey patch," M. canis
2. Endothrix -- "black dot," T. violaceum, T. tonsurans 3. Endothrix -- "favic," T. schonleini |
|
|
What causes tinea cruris?
|
E. floccossum, T. rubrum
|
|
|
What causes tinea corporis>
|
T. rubrum, T. mentagrophytes
|
|
|
How to diagnose dermatophyte infections?
|
Microscopically -- skin/hair/nail, digest with KOH/ink/calcofluor staining
Culture -- Add cycloheximide chloramphenicol (slow, sensitive to growth conditions) |
|
|
What is the life cycle of aspergillus fumigatus?
|
1. spore germinating
2. initiating hypal growth 3. form mycelium 4. form foot cells 5. create canidiophores that form spores |
|
|
What do dermatophytes secrete?
|
Urease. Will turn urine from yellow to red.
|
|
|
Where are aspergillus usually found? What are some special characteristics?
|
Rotting vegetation.
Asexual mold with septate hyphae. Temperature tolerant. |
|
|
What are some conditions often confused with dermatophytes?
|
a. tinea versicolor- sun spots (caused by yeast colony Malassezia)
b. sporotrichosis – granulated c. cutaneous candidiasis – entire area (not just edges) are inflammed d. seborrheic dermatitis -- scaling and separation of skin on scalp e. bacterial impetigo- staph/strep f. atopic dermatitis- eczema – exaggerated immune response g. Psoriasis- prolifreaiton of keratinocytes |
|
|
What types of aspergillus cause infections?
|
1. fumigatus (causes most infection)
2. terreus 3. flavus 4. niger |
|
|
What is sporotrichosis?
|
Subcutaneous. Granulated so hard to be treated with antifungals. Slow, painless, caused by sporothrix schenkii (dimorphic yeast).
Papule reddens, pustular, ulcerated Treated with surgery. Indicators on USMLE: Anyone dealing with gardening, agriculture, landscaping, etc Cats Cat |
|
|
What is allergic bronchopulmonary aspergillosis? How does it manifest? How is it treated?
|
Found in hypersensitive body
1. Rasied IgE and Eosinophils 2. Treated with inhaled corticosteroids 3. Can cause fibrosis of lungs |
|
|
What is chromomycoses?
|
Black soil fungus
Causes warts Found in tropics Liquid N2 |
|
|
What is aspergilloma? Where is it found? What are symptoms?
|
1. Found in preexisting balls in lung, encapsulated (cannot spread)
2. Usually asymptomatic but can cause spitting of blood |
|
|
What is mycetoma?
|
“madura foot”
i. 60-70% caused by actinomycetes 1. others caused by fungal infections (different species) ii. drain fluid from them grainy fluid (black/light color) iii. treatment: anti-fungals 1. usually don’t work so require amputation |
|
|
What is invasive aspergillosis? Where does it spread?
|
Found in immunosupressed bodies.
1. Small spores and can be carried to the alveoli of lungs where they can swirl and germinate ("spaghetti strings" of hyphae) 2. Can be pulmonary or disseminated 3. Fatal, but mortality rate has decreased b/c of early detection 4. Difficult to treat |
|
|
What is needed for differentiation of TH0 to TH1?
|
IL-12 and IF-gamma are needed.
|
|
|
How can an aspergillus infection be identified? Describe what can be seen with each method.
|
1. biopsy and microscopic examination
2. culture (problem of contamination!) 3. CT scans -- unique halo appearance 4. PCR assays 5. Galactomanan ELISA assay -- complex sugar secreted by fungal cell wall of aspergillus |
|
|
What is needed for differentiation of TH0 to TH2?
|
Cell needs IL4.
|
|
|
What does a CT "Halo" sign indicate?
|
Early stages of invaseive aspergillosis.
Later stages, see "air crescent" because necrotic mass has reduced in size. |
|
|
What does IL-10 do?
|
It inhibits the immune response.
|
|
|
How can invasive aspergillosis be treated?
|
1. Amphotericin B (wide spectrum agent)
2. Lipid AMB (less side effects) 3. Voriconazole or Caspofungin (not effective against zygomycetes) 4. Combination therapy (developing) |
|
|
Lack of the gamma chain leads to?
|
SCID. This is because TH1 TH2 and T-cytotoxic cells can not respond to cytokines.
|
|
|
How can invasive aspergillosis be prevented?
|
1. Hepafilter
2. Patient Monitoring 3. Poscanazole prophylaxis |
|
|
Which immunoglobulins have the hinge region?
|
IgA, IgG, and IgD. Also have three constant regions.
|
|
|
What are zygomycetes?
|
1. Primitive, broad irregular non-septate hyphae family
2. Known as "bread molds" |
|
|
Which immunoglobulins do not have a hinge region, but instead have a fourth constant region?
|
IgM, IgE.
|
|
|
The flexibility in the hinge region is due to which amino acid?
|
Proline
|
|
|
How do zygomycetes reproduce?
|
1. Asexually -- sporangia filled with sporangiospores (when ready, sac bursts)
2. Sexually -- Zygospores; 2 colonies send out hyphae that fuse (nuclei fuse to doen transient diploid which undergoes mitosis to form haploid spores) |
|
|
What is the FAB fragment responsible for?
|
Binding of the immunoglobulin to the target.
|
|
|
What are the disease characteristics of zygomycosis? Who is it commonly found in? What clinical manifestations are common?
|
1. Rare
2. Found in diabetics, burn victims, leukemics, dialysis 3. Rhinocerebral: dischange, swelling, numbness, necrosis; pukonary, cutaneous, GI, disseminated 4. 50-80% mortality rates |
|
|
What is the J-chain?
|
The part of IgM or IgA that links all parts of the immunoglobulin together through the Fc Receptor.
|
|
|
How can zygomycosis be treated?
|
1. Correction of underlying disease sympthoms
2. Surgical debridement of necrotic tissue 3. Amphotericin B 4. Lipid AMB |
|
|
What are ITAMs?
|
ITAMs interact with several members of the SRC family of tyrosine kinases, activated enzymes phosphorylate tyrosine residues on cytoplasmic tails of the heterodimers, alters gene expression.
|
|
|
How to diagnose zygomycosis?
|
1. Microscopic examination
2. Culture 3. CT scan |
|
|
What is candida? Half of infections are caused by which? What are some special characteristics?
|
Ascomycetes related to baker's yeast. 9 species are pathogenic to humans.
50% by C. albicans Most dimorphic (can spread around the body easily and burrow inside) |
|
|
What is pneumocystis carinii? How to diagnose? How to treat?
|
1. Ascomycete in a unique class.
2. 85% of AIDS patients infected (pneumonia, fever, non-productive cough, shortness of breath, weight loss & night sweats) Diagnosis: Sputum, bronchoscopy, silver/Giemsa, CFW Treatment: Antifungals ineffective, TMP/SMX, or pentamidine |
|
|
Where are Candida species found?
|
Human Commensal. Normal flora.
1. Skin 2. Mucous membrane 3. Mixed biofilm |
|
|
What are symptoms of viral hepatitis?
|
Fever
Fatigue RUQ pain Malaise Anorexia Jaundice |
|
|
What are some cutaneous candidiasis?
|
a. chronic mucocutaneous candidiasis immune compromised from birth
i. suffer recurrent candida infections ii. extreme cases form granulomas b. Paronychia and onychomycosis – skin glistens c. Intertrigo- common in obese people i. fissures and cuts form on skin and colonization of candida d. Diaper rash- caused by bacterial infection or candida infection e. candida balanitis penial infection i. especially in uncircumcised people ii. unprotected sex with women with candida vaginitis |
|
|
How does viral hepatities affect pathology of the liver?
|
1. hepato-cellular injury—necrosis of hepatocytes
2. infiltration of mononuclear cells 3. hyperplasia of kupffer cells 4. cholestasis |
|
|
What disease forms occur in viral hepatitis?
|
1. Acute viral hepatitis
2. Sub-clinical acute viral hepatitis (virus enters liver, replicates and induces immune response but it is so mild, here are no symptoms) 3. Fulminant viral hepatitis - mass destruction of liver and altered brain function 4. Chronic viral hepatitis 5. Cirrhosis and cancer (<--caused by HBV and HCV) |
|
|
What are some types of mucosal candida?
|
a. Oral Candida “thrush”- see spots on palate and tongue
i. grows in plaques cottage cheese like chunks made of a mixture of candida immune cells, dead epithelial cells… 1. hypae digging into the epithelia ii. palate candidiasis seen under improperly cleaned dentures b. Candida esophagitis – same creamy patches i. common in aids patient ii. cause difficulty swallowing c. Mucosal Candida GI tract- in immune compromised patient (leukemia, s/p chemo) d. Candida Vaginitis- infects vulva and vagina i. strong inflammation, itching, creamy cottage-cheese like d/c with a characteristic type odor (yeast-like odor) ii. often misdiagnosed with trichomonas, bacterial vaginosis (smells worse the candida) Chlamydia, Gonorrhea e. Urinary tract candidiasis |
|
|
What are biochemical markers for hepatitis?
|
Signs of liver inflammation (not virus specific)
1. elevation of amino-transferases (ALT, SGOT) 2. rise in serum bilirubin (dark urine) |
|
|
What can cause disseminated candidiasis?
|
1. Tubes and shunts
2. Chemo 3. Invasive surgery 4. Burns and trauma |
|
|
How to make differential diagnosis of different forms of hepatitis?
|
1. Route of infection
2. Length of incubation period 3. Serological tests -- finding specific viral antigens or antibodies using ELISA or PCR |
|
|
Why is disseminated candidia so dangerous?
|
It can infect brain, heart, GI, kidneys, kidney, and spleen. Can lead to endocarditis (likes to stick, especially to the valve and can get together with bacteria. Can also lead to embolism)
|
|
|
What classification does Hep A belong to? What is its nucleic acid structure? How is it transmitted? How is its onset? Where does it replicate?
|
Picorna. +ssRNA.
Fecal-Oral. Abrupt. Cytoplasmic replication. |
|
|
How do you verify that you have a candida infection?
|
1. Sample collection, staining, microscopy, culture (can't differentiate)
2. Chromagar (differentiate through colors but expensive) 3. Carbon assimilation 4. Immunodiagnostic kits (good for systemic disease, contains Ab directed against fungal antigens) 5. DNA techniques |
|
|
What is the structure of HAV? What is the pathogenesis of HAV?
|
Picorna, +ssRNA, naked, icosahedral
1. Oral 2. Intestinal 3. Via blood to liver 4. Virus produced in liver, secreted into bile and in stool. |
|
|
How are candida infections treated?
|
Cutaneous -- use topical azole cream
Mucosal -- mild: creams severe/deep internal: systemic treatment |
|
|
What is the time course of HAV infection?
|
1. Shorter incubation -- 2-4 weeks (already replicating, can be found in feces)
2. Acute Phase symptoms -- 1 month after infection (uncommon icteric and elevated serum liver enzymes) 3. replication of virus in liver induces CMI and antibody responses (IgM, IgG) |
|
|
What is cryptococcus? What are some characteristics?
|
Another cause of opportunistic infection.
Encapsulated yeast, no hyphae. 2 varieties: neoformans, gattii |
|
|
How does HAV affect the immune response?
|
1. Interferon limits viral replication
2. NK cells and cytotoxic T cells are required to lyse infected cells antibody 3. Complement and ADCC facilitate clearance of virus |
|
|
What are cryptococcus' three main virulence factors?
|
i. encapsulated- protection against phagocytosis
1. made of gluco/galactomannan 2. offensive --> “capsule shedding” – shed bits of capsule misleading our immunes system 3. induced by high CO2 and low iron (as in human body) a. make thicker capsules in the body then out 4. evolved to have a capsule soil ameba engulf fungi (like macrophages) a. by developing capsule can overcome enemy in soil ii. melanin- cell wall is rich in melanin that protects against oxidation attack from macrophages iii. thermotolerant- can grow at body temperature |
|
|
What are the viral factors of HAV and HEV? How is it transmitted?
|
1. Capsid viruses are very resistance to inactivation
2. Contagious period extends from before to after symptoms 3. Virus may cause asymptomic shedding It can be transmitted via fecal-oral, ingestion of contaminated food and water |
|
|
What happens when cryptococcus is engulfed by macrophages?
|
reproduces in macrophages until it lyses and spreads the fungi in the body
|
|
|
Who is at risk for HAV and HEV? How can it be controlled?
|
People in overcrowded, unsantiary areas.
Control through hygiene and killed vaccine. |
|
|
What are unique features of hepandavirus?
|
1. Enveloped virions containting partially DS, circular DNA genome
2. Replication is through RNA intermediate. Encodes and carries reverse transcriptase. 3. Encodes special surface envelope proteins |
|
|
How to identify crypt?
|
1. India ink prep of CSF
2. Latex agglutination assay 3. Culture and india ink prep |
|
|
What is a Dane particle? What is it comprised of? What is special about each component?
|
HBV virion. Binds to the host cell via the preS domain of the viral surface antigen, internalized by endocytosis.
Comprised of (in to out): 1. dsDNA (+strand partially complete) 2. DNA polymerase (reverse transcriptase ability) 3. icosahedral capsid 4. proteins 5. envelope |
|
|
How can crypto be treated?
|
1. Amphotericin B (10 weeks)
2. Amphotericin + 5-flucytosine (6 weeks) 3. AMB + 5FC (2 weeks) then fluconazole (3-6 months) |
|
|
What is the difference b/w acute and chronic viral hepatitis? Which heps cause chronic? Why is chronic hard to diagnose?
|
Acute: first, flu-like symptoms, then jaundice, enlarged liver, high blood levels of ALT, GGT, alkaline phosphatase.
Chronic: Patient often asymptomatic w/enlarged tender liver and mildly elevated liver enzyme. |
|
|
Explain B-cell cross-linking?
|
Occurs when an antigen binds two neighboring receptors at once, it activates a tyrosine kinase pathway.
|
|
|
What is the structure of HBV?
|
Hepadna, ds circular DNA, enveloped icosahedral capsid
|
|
|
How do thymus independent antigens activate B cells?
|
By binding to the receptors on the B cell other than the IgM/IgD receptors. This induces B-cells to undergo polyclonal activation.
|
|
|
How is the DANE particle shed into blood? What is important about these structures?
|
1. Spherical particle HBcAg (small) -- contains just surface proteins. Anti-HBcAg does not result in immunity.
2. Filamentous particle HBsAg-- formed by aggregation of small spherical particles (contains all surface proteins). Important because antibodies against this component are protective (immunity). |
|
|
What is a common B-cell mitogen?
|
LPS – lipopolysaccharide. From gram positive bacteria.
|
|
|
What is a marker for active, highly infectious HBV? How does it affect pregnant mothers?
|
HBeAg. This is the soluble component of HBcAg.
Pregnant mothers with HBeAg will almost always transmit HBV to offspring. |
|
|
Where does T-cell dependent activation of B-cells take place?
|
In secondary lymph tissue.
|
|
|
What disease states are caused by HBV? What are complications?
|
1. Acute hepatitis
2. Fulminant hepatitis -- severe acute hepatitis with rapid destruction of the liver. 3. Chronic hepatitis 4. Co-infection w/HDV Complication: primary hepatocellular carcinoma, cirrhosis |
|
|
The process of fusion of immunoglobulin genes that codes for one gene and creates one receptor or antibody is called?
|
VDJ recombination
|
|
|
What are the three states of chronic hepatitis?
|
1. Asymptomatic carrier: patient never develops antibodies against HBsAg and harbors virus w/o liver injury
2. Chronic-persistent hepatitis: patient has low-grade "smoldering" hepatitis 3. Chronic active hepatitis: acute state continuing w/o normal recovery |
|
|
What are RAG1 and RAG2?
|
Lymphocyte specific enzymes that binds to the V and J regions arbitrarily in the gene, cuts the DNA, and then the VJ regions are ligated.
|
|
|
How to diagnose HBV?
|
Serology:
1. HBsAg (live infection) 2. IgM anti-HBcAg (new infection) 3. IgG anti-HBcAg (old infection) 4. HBeAg (high infectivity) 5. anti-HBeAg (low infectivity) |
|
|
The T-Cell receptor is made up of?
|
Alpha-beta, or Gamma-delta chains, with alpha-beta being most common.
|
|
|
How to prevent and treat HBV?
|
1. Recombinant vaccine
2. Lamivudine (anti-HIV supresses HBV, though there is relapse) 3. Inferon-alpha (supression and prevention of cirrhosis) |
|
|
What creates the diversity in T-cell receptors?
|
Combinations of alpha-beta chains by the same RAG mechanism.
|
|
|
Describe the defining characterisitics of HCV and HEV.
|
1. Falvivirus, ssRNA, envelopoed. much like HBV, leads to cirrhosis and liver failure
2. Calcivirus, ssRNA, naked. much like HAV |
|
|
What is another way to increase genetic variability?
|
Altering of the reading frame of the DNA.
|
|
|
What is HDV? What is its structure? How does it work? How is it transmitted?
|
Incomplete RNA virus transmitted parenterally and can only replicate with the help of HBV. Steals HBV's envelope HBsAg in order to cause infection.
|
|
|
What is unproductive combination?
|
When fusion of the VJ sequence alter the reading frame so that no product is made.
|
|
|
What are the ways in which HDV infects? Who is affected? How does the body react?
|
1. Co-infection: HBV, HDV presented together, causing acute hepatitis. Anti-HBsAg = immunity to both.
2. Superinfection: HDV infects a person who has chronic HBV infection, resulting in acute hepatitis in already chronically ill. Leads to higher incidence of fulminant hepatitis, cirrhosis, greater mortality. Patient cannot manke anti-HBsAg. |
|
|
Where do the RAGs bind?
|
To RSS (recombination signal sequences) that flank the V and J regions.
|
|
|
How are each of the hepatitis viruses replicated?
|
LOOK THIS UP!
|
|
|
What 4 species of dimorphic fungi exist? What are the defining characteristics of each? Are these virulent?
|
1. H. Capsulatum -- mold form, – round terburculate macro-conidia and has microconidia (infective part- usually when disturbed natural surrounding of mold); once in lung, form small yeast cells
2. B. Dermatitids -- mold (microcondiia); yeast in body (large multi-nucleated) 3. P. Brasilliiensisi -- mold (nothing special); yeast (large cell with babies budding off -- ship wheel appearance) 4. C. Immitis -- mold (phallic arthrospores); yeast (large multi-cell spherules) All of these are inherently virulent. |
|
|
Why does mutation in the DNA repair system cause immunodefiency?
|
Because the same machinery is required for the development of lymphocytes.
|
|
|
When heavy chains begin to arrange what happens first?
|
D and J segments are arranged on both chromosomes.
|
|
|
5% of people who inhale condida may suffer from...? What are the symptoms?
|
Threatening severe pneumonia.
Can have calcifications and granulomas, which can remain dormant for many years. Weakened immune system re-activates. |
|
|
If the VDJ segment on the first chromosome is unproductive what happens?
|
The VDJ segment is formed on the second chromosome.
|
|
|
Where are infections of histoplasmosis commonly found? Who is infected? How does it work? What are symptoms?
|
Found in Mississippi Valley from bird/bat droppings.
Those with t-cell dysfunction are susceptible. T cells activate macrophages, and these will kill the disease. Clinical manifestation in AIDS patients, presentation of granulomes, ulcers, sores. Can disseminate throughout body, often mistaken for TB. |
|
|
If the VDJ segment on the second chromosome is unproductive what happens?
|
The cell undergoes apoptosis.
|
|
|
What is the infective process of histoplamosis?
|
1. Fungal form grows under bird/bat colonies, and macro/microcondida are formed.
2. These are inhaled. 3. In lungs, turn into yeast., leading to phagocytosis by the macrophages. 4. The yeast replicate, causing lysis of macrophages. The yeast have ability to inhibitacidification and digestion by the phagolysozyme |
|
|
If the kappa gene rearrangement on the first chromosome fails, what happens?
|
There is kappa rearrangement on the second chromosome.
|
|
|
What is the most virulent species of fungus in the world? What is its shape? Where is it endemic? What are symptoms?
|
Coccidioides immitis. Alternating barrel-shaped arthrocondidia.
Pneumonia-like symptoms. Endemic in arid SW US, Mexico, Argentina. |
|
|
What are the clinical findings of Coccidiodomycosis? How does it spread within the body?
|
Find spherules in tissues.
Rash --> ulcerations --> biopsy, see yeast w/sperules surrounded by a capsule that protects them. Grow in capsule until they reach capacity, then spherule bursts and infects somewhere else. Macrophages that swallow them become disseminating agent in the body (to joints and even brain) |
|
|
If the kappa rearrangement on the second chromosome is unproductive, what happens?
|
The cell undergoes apoptosis.
|
|
|
What is paracoccidiodomycoses? What does it look like? Where is it endemic? What are clinical findings? How is it treated?
|
Skin, mucous membranes, lungs infected with ulcers. In yeast form, looks like a ship's wheel. Endemic in South America, especially in males.
Treated with Sulfas, azoles, and AMB. Sulfas work by inhibition of biosynthesis of folate (vitamin B12). |
|
|
Where is pennicillium marneffei found? What does it cause? Who is most susceptible? Is it treatable?
|
Found in Thailand (Far East), especially in AIDs patients. Causes Pneumonia (fever cough) with system lesion formation
Is treatable! |
|
|
What is somatic mutation?
|
Mutations in the gene that produces the receptor that leads to greater affinity for the antigen.
|
|
|
How does the immuno-diffusion test work? What types results are been? What do they mean?
|
slab of agar punch holes in it and put in the center histoplama antigens
i. in the wells around put patients serum or urine get slow diffusion of proteins from histoplasmin toward the outer wells ii. otherside get migration of antibodies meet and cause precipitation (conjugate formation) 1. 2 types of bands: a. H band close to the well – found in any person previously exposed to histoplasma i. H antibodies last for a very long time b. M band (antigen) closer to the well- suggestive of current infection i. M antibodies only exist in body for a few month after exposure |
|
|
What are accuprobe fluorescent rRNA DNA probes? How does it work?
|
kit that contains fluorescent DNA probes
i. interact with rRNA of histoplasma causing the probe to interact with it conjugated to a fluorescent molecule 1. positive interaction get a fluorescent color in spectrometer 2. VERY SPECIFIC – and can be used on very small samples |
|
|
Where does somatic mutation take place?
|
In the germinal center of the lymph node.
|
|
|
What are accuflorescent RNA probes? How do they work?
|
kit that contains fluorescent DNA probes
i. interact with rRNA of histoplasma causing the probe to interact with it --> conjugated to a fluorescent molecule 1. positive interaction get a fluorescent color in spectrometer 2. VERY SPECIFIC – and can be used on very small samples |
|
|
When does the germinal center of the lymph node form?
|
Only after exposure to an antigen.
|
|
|
What is the criteria for selecting a good antifungal target? What are some problems?
|
1. Nucleic Acid Synthesis inhibition (not specific; b/c a fungi is euk., if it hurts them, it prob. hurts us)
2. Nuclear division inhibition (also inhibits nuclear division, narrow therapeutic window with lots of side effects) 3. Interfere with membrane function 4. Interferes with ergosterol synthesis 5. Interfere with cell wall synthesis |
|
|
What is affinity maturation?
|
The receptor with the highest affinity for the antigen will be the one to develop. The process takes weeks.
|
|
|
How does Griseofulvin work? What are its actions? How is it isolated? Which fungi does it affect? Where is it localizaed? How is it used?
|
Isolate from pcn griseofulvin. Binds mictrotubules, inhibits mitosis.
Works on dermatophyets only, especially captitis. Doesn't spread well into different compartments of body, so it localizes in skin and scalp. Treatments take much longer than with azoles; however, they are much cheaper. |
|
|
What are the two consequences of the deamination of cytidine?
|
1. Cytidine is produced instead of tyridine by a point mutation, so that the next DNA synthesis will have CG instead of TA.
2. It will replace T with C and DNA breaks are made by the DNA repair machinery. |
|
|
In terms of antibiotics, what are the cell wall inhibitor families?
|
1. Beta-lactams: Penicillins, Cephalosporines, Monobactams, Carbapenems
2. Glycopeptides: Vancomycin 3. Others: Bacitracin, Cycloserin, Fosfomycin |
|
|
What are the structures of polyenes? How do they work?
|
8 molecules form circular pore in membrane of fungi, leading to leakage and death by leakage. Binds preferentially to ergosterol (efficient hydrogen bonds).
|
|
|
What is the first stage of PDG formation?
|
Precursor formation:
Occurs in the cytosol Uridine diphosphate (UDP)-NAM-pentapeptide and UDP-NAG are synthesized Antimicrobial agents which interfere with these steps: fosfomycin, cycloserine |
|
|
What enzyme performs the class switch?
|
The same enzyme which does the point mutation during somatic hypermutation.
|
|
|
What is the second stage of PDG formation?
|
Polymer formation:
The precursor molecules are transferred to a lipid carrier in the cytoplasmic membrane: phosphorylated undecaprenyl alcohol, which allows for transport of the subunits across the hydrophobic interior of the cytoplasmic membrane to the outside surface |
|
|
What is Nystatin? How is is made? How is it used? How does it work?
|
It is made from streptomyces noursei. Topical use only! Put into mouthwash for oral thrush.
In liposomal form, less toxic, which may lead to systemic uses for this drug in the future. |
|
|
How does bacitracin work?
|
Bacitracin a peptide antibiotic that interacts with the carrier, preventing dephosphorylation of carrier, or transport from the precursor to the nascent peptidoglycan
|
|
|
Where does V(D)J recombination take place?
|
In the bone marrow
|
|
|
How do transglycosylases and transpeptidases work?
|
At the cell surface peptidoglycan units are reticulated by the action of transglycosylases (catalyzing the polymerization between sugars) and of transpeptidases (catalyzing the polymerization between peptidic chains)
|
|
|
How is AMB given? What are the side effects?
|
Given as suspension with detergent through IV.
Immediate SE: chills, fever, nausea, phlebitis Long Term SE: anemia (lyses RBC + WBC), nephrotoxic |
|
|
How do beta-lactams work? Glycopeptides?
|
β-lactams inactivates transpeptidases
β-lactams are analogs of D-Ala–D-Ala and suicide substrates for transpeptidases Glycopeptides binds to D-Ala–D-Ala termini and thus inhibits the action of transglycosylases and transpeptidases |
|
|
What happens during V(D)J recombination?
|
One fragment of V, D, and J are selected from their pools of gene fragments and are combined to form a single variable (V) – region exon.
|
|
|
Describe HIV structure. What are its components? What makes them? What are their functions?
|
1. Env gene makes envelope proteins GP120 (attachment proteins that interact w/CD4) and GP 41 (transmembrane protein holding GP 120 in envelope)
2. Gag sequences code for proteins inside envelope p17 matrix, p24 capsid, p7p9 nucleocapsid 3. Pol genes make integrase, protease, RNA, RT |
|
|
What is liposomal AMB? What does it do? What is its structure? What are the advantages and disadvantages of using it?
|
synthetic bi-layers where AMB can be carried inside capsule
1. not free to circulate and damage our body 2. liposomes have high affinity to fungal cell wall stick to it, liposome breaks apart and AMB can work on the fungus 3. advantage: a. easier administration LESS SE (renal and infusion toxicity) b. can increase concentration of drug you give the patient 4. disadvantage – COST ($1000/day for a few weeks) |
|
|
What is the difference b/w HIV 1 and HIV 2? How do they work? Who do they effect?
|
1. HIV1- high homology with SIV infects chimps; more aggressive biological behavior than HIV 1
2. HIV2- high homology with SIV that infects another monkey i.homology between HIV1+ HIV2 only 45% but both have homology to their respective SIV up to 85% ii.transmitted less efficiently by homosexual intercourse + from mother to child iii.rate of CD4 decline less + lower viral burden iv.replication in cell is slower/less efficient v.less cytophathic in cell culture vi.usually limited to African countries vii.doesn’t contain vpr but does contain vpx |
|
|
What is infectious mononucleosis?
|
An inflammatory disease that affects many lymphocytes and protects them from apoptosis. It is a polyclonal disease.
|
|
|
What are the regulatory genes in HIV? What do they do? Give a brief, general description of each.
|
1. LTR -- integration w/sticky ends & viral gene expression w/promoter/enhancer function
2. Tat -- potent promoter transactivator of transcription (upregulation); spliced gene 3. Rev -- regulated transport of un/spliced transcripts to cell cytoplasm; spliced gene. Decreases CD4, MHC I expression on host cells; binds to env to decrease splicing 4. Nef -- manipulates T cells; required for AIDS 5. Vif -- virus infectivity and assembly 6. Vpu -- virus assembly and release; also decreases CD 4 expression, unique to HIV 1 7. Vpr -- transports viral DNA into cell nucleus, arresting host cell growth 8. Vpx -- gene is found in HIV-2 and SIV |
|
|
What are flucytosines? How is it activated? How does it work? What are side effects? What it
|
Can only be activated by cytosine deaminase (only present in bacteria and fungi).
Incorporated into RNA as fluorocytosine. Incorporated into DNA, DNA synth blocked. Causes the death of WBCs. Narrow effectivity b/c of point mutations and resistance. Usually given in combination with AMB |
|
|
What are the coreceptors for macrophages and T-cells of HIV-1? What uses them? How are they used?
|
a.CD4-R
b.co-receptor along with CD4 receptor enables viral entry into the cell i.receptor used by chemokines 1.alpha chemokine receptor expressed in T cell lines a.Cxcr4- infected by x4 virus 2.beta chemokine receptor expressed on macrophages a.Ccr5- infected by R5 virus ii.some viruses recognize more efficiently the alpha- others the beta c.activated T cell--> expresses both alpha and beta chemokine receptors (so can be infected by both types of viruses) |
|
|
B- Cell lymphoma is what?
|
Malignancy is usually clonal, usually originates in one cell.
|
|
|
What is the life cycle of HIV?
|
1. Virus uses CD4, CCR5, CXCR4 to attach to host
2. Gets inside, reverse transcriptase transcribes mRNA to dsDNA 3. Integrase into host cell chromosome, develop provirus. Virus can remain as an integrated virus for a long time (can also stay as episomal cytoplasm virus for a long time as well) 4. Transcription leads to unspliced (+ssRNA, genome new virus) and spliced (structural proteins) |
|
|
What are 2 ways by which viruses contribute to carcinogenesis?
|
a. Expression of viral genes- that bypass the need for one or more of the steps that would normally be involved in multi-step carcinogenesis
b. Stabilizing or promoting- the growth of a cell population in which oncogenic genetic change may occur |
|
|
Where are viral components produced in HIV? How will they be used?
|
Accumulation near plasma membrane
1. Viral glycoproteins that are produced using ER cellular enzyme will be inserted into cellular membrane and accumulate 2. During enveloping of viral components, budding of viral particles. First buds contains RNA and viral polyproteins--> not a mature particle. Needs activity of viral protease to cleave viral proteins--> occurs in the virus outside the cell. |
|
|
What are viruses that are associate with causing cancer?
|
DNA:
1. HBV 2. HPV 3. EBV 4. Kaposi's Sarcoma 5. HHV8 RNA: 1. HCV 2. Human T cell leukemia virus |
|
|
What mutation leads to immunity to HIV? Slow progession of disease? Rapid progression?
|
1. Homozygous CCR5
2. Heterozygous CCR5 3. CXCR1 mutation |
|
|
Explain the structure of the poly-Ig receptor and where is it found?
|
It is composed of 5 Ig domains, and is found on basolateral mucosal epithelia.
|
|
|
What is the clinical progression of AIDS? What does the course follow?
|
1. Asymptomatic
2. Persistent generalized lymphadenopahty 3. Symptomatic 4. AIDS-defining conditions Follows decline in CD4+ T cells. |
|
|
What are differences b/w DNA and RNA oncogenic viruses?
|
1. DNA viruses do not always induce cancer in native hosts (e.g. SV40/adeno)
2. RNA viruses and SOME DNA (HPV) induce. 2. Transformation cycle (integration) vs. productive cycle (no integration, DNA) 3. Viral transforming genes (not host derived DNA or host derived RNA) = early genes. |
|
|
What are three known mechanisms of T cell death during HIV?
|
1. GP160 in T cell membrane binds to adjacent CD4 receptors on same T-helper cell membrane, tearing T-cell membrane, destroying cell.
2. GP160 in infected cells binds to other CD4 T helper cells, resulting in cell to cell fusion (up to 500 cells!), creating multinucleated giant cells, avoiding blood contact. 3. GP160 can mark T cells for destruction by cytotoxic CD8 T lymphocytes. |
|
|
What part of the antibody binds to the receptor?
|
The Fc portion.
|
|
|
How does HIV affect B cells?
|
Polyclonal activation, resulting in hypergammaglobulemia = diminished ability to produce antibodies in response to new antigens or immunization
|
|
|
IgE mediates which hypersensitivity reactions, and what is used to test for an inflammatory response?
|
Mediates: hay fever, asthma, hives, anaphylaxis
PK reaction on skin used to test for inflammatory response. |
|
|
How does HIV affect monocytes and macrophages?
|
They are not destroyed but serve as resevoirs for HIV as it replicates. Migration across blood-brain barrier.
|
|
|
What is HPV? What is the structure? What does it cause? Where does it infect? What forms are there?
|
small, nonenveloped DC circular DNA virus. Infects skin and mucus.
1. HPV 1 + 6 create begign head & neck tumors, can also cause benign cervical lesions 2. HPV 18 + 16 can cause pre-malignant cervical intraepithelial lesions. Transmitted through sex. |
|
|
Go through the time course of HIV. What are the clinical stages? What can be seen?
|
1. In the beginning, spike in p24 (peak of viral overload -- 4-8 weeks). Immune system does its job for a while.
2. Asymptomatic period. Virus chased into lymph, trapped in dendritic cells. 3. Cells will be infected, lymph will be degenerated. Virus will reappear in the blood stream. 4. CD4+ T cells die. Below 200 is AIDS (after 8 years). Decline of Ab to p24. |
|
|
When IgE binds to the Fc receptor on basophils and mast cells it has no effect when it is monomeric. What has to happen in order for IgE to cause granule release?
|
When an allergen binds to the FAB portion of the IgE it causes cross linking of the Fc receptors. The cross linking causes exposure and release of the granules.
|
|
|
How does HIV escape both the CMI and humoral response? Why?
|
1. Humoral antibodies- not very efficient against the virus b/c:
i.antigenic drift of gp120- RT is not a very accurate process (high rates of mutations – change in glycoproteins) ii.heavy glycosylaiton of gp120- glycosylation impedes activity of antibodies iii.spread from cell to cell – by cell fusion without entering the blood itself 2. Cellular immunity: i.virus infects CD4 cells and impairs them ii.virus hidden from Th cells iii.sequestration (like in CNS cells) iv.latent provirus- not viral antigen production v.impairs CTL, leading to down regulation of HLA class I, low perforin, epitope mutation or deletions vi.CTL exhaustion--> CTL killed due to infection by virus (ie. FAS CTLs killed by FAS-L expressed on infected cells) |
|
|
What is the association b/w HPV and cancer"Why are we blaming viruses for cancer?"
|
High risk HPV viral genome in more than 97% cervical cancer. High risk HPV cell transform cells in vitro.
Certain types of HPV (high risk) associate with cancer. High risk oncoproteins bind to and inactivate tumor suppressor proteins. Prevalence of cancer-assoicated HPV types increases with grade of squamous intraepithelial lesions. |
|
|
What are four ways in which HIV can be treated? What are their mechanisms? What are examples of each? What are problems with them?
|
1. Reverse transcriptase inhibitors -- work on cirus after entry on replication state of virus.
a) Nucleoside analogs: Has to be phosphorylated by cellular enzymes. Inhibits activity of RT when incorporated into elongated chain. (AZT, ddl, 3TC, d4T, ddc) b) Non-nucleoside: Bind to RT. (delaviridine, nevirapine) 2. Protease inhibitors -- work on inhibiting cleavage of gp and gag proteins; mimick target site of cleavage. (Indinavir, nelfnavir, ritoavir, saquinavir) 3. HAART -- drug cocktails, each w/different mechanisms of action. Usually 2 nucelosides, 1 protease inhibitor e.g. AZT/3TC/indinavir 4. Fuzeon |
|
|
How do B-cells that are already specific to IgE proliferate?
|
IL-4 released by TH2 cells allows them to proliferate.
|
|
|
What are the methods of lab analysis for HIV? When is each used? What are we looking for?
|
1. ELISA -- initial screening, but many false positives.
2. Western Blow -- confirmation; shows all viral Ag on a strip 3. IF -- also confirmation 4. RT-PCR -- detection of virus in blood (p24 Ag; p24is an early marker of infection) 5. CD4:CD8 ratio -- evaluation of progression, monitor HIV status 5. |
|
|
Describe the steps of HPV genome organization.
|
1. Virus has a has LCR, which contains promoters of virus and regulatory sequences (initiate replication and transscription)
2. Virus infects basal cells of the mucosa (replicating later) 3. Replicates an episome (not integrated into host genome) |
|
|
What factors of produced in the host lead to slowing HIV infection? Facilitating HIV infection?
|
1. HIV specific immune response, CD8 T cell derived suppressor proteins (Caf), inhibitory cytokines
2. Inflammatory cytokines, enhancing and interfering Ab, cellular activation |
|
|
What are the early genes in HPV 16 genome organization? What are the late genes? What do they do>
|
1. E1 --> initiate DNA replication of virus
2. E2 --> necessary to control transcription 3. E4 -->responsible to disrupt cytoskeleton and allow virus to be released 4. E6, E7, E5--> transforming proteins and have some function during productive replication of virus 5. ” L1+ L2- encode viral capsid proteins |
|
|
What are some alternative approaches to vaccination against HIV? Describe them. How do they work? Which ones have been successful?
|
i.envelope glycoproteins- produced by gene cloning in mammalian or insece cells--> failed trial stopped
ii.recombinant live virus- incorporating genes fro HIV envelope gp and perhaps other proteins iii.DNA vaccine- 1.combination of structural proteins –Gag + Env--> initial trials failed b/c saw that there was escape from CTL responses 2.new vaccines based on nonstructural proteins Tat, Rev, Nef 3.live attenuated deletion mutant- NEF gene-unsafe 4.chimeric SIV-HIV – IL2 DNA vaccine- contains genes from SIV (monkey virus) in which the envelope virus of HIV was cloned |
|
|
Paracytosis through the mucosal membrane involves what immunoglobulins?
|
IgA and in some cased IgM
|
|
|
What are problems with vaccine development for HIV?
|
i.we are not very clear on which antigens elicit the immune response
ii.HIV expresses antigenic heterogeneity iii.mucosal immunity may be needed iv.enhancing antibodies exist v.viral genome integrates into host cell chromosome vi.major target of HIV is the immune system – but we need the immune system to fight the virus |
|
|
How does HPV 16 infect the basal cells of the mucosa? How dies it replicate?
|
1. maintenance replication --> initial replication enables to pass viral genome into daughter cells
2. productive replication --> when basal cells are triggered to differentiate they detach from the basement membrane and move upward a. during this movement there are changes in the epithelial cell structure and proteins --> differentiated layers 3. in order for virus to replicate it own genome --> need differentiated (granular layer) cell environment a. but also need DNA replication machinery but this is stopped when the cell is differentiating i. virus encodes its own proteins (genes like E6 and E7) allow to reactivate synthesis of DNA in differentiated cell b. viral reproduces genome and capsid proteins will be made in the granular and corneum layers --> assembled and virus released by natural all of epithelial cells (Sloughing) |
|
|
What are the differences b/w sCJD and vCJD? Median age of death? Median duration of illness? Clinical signs and symptoms? Electroencephalogram? MRI? Histological analysis?
|
1. Classic -- 68 years death; duration 4-5 months; dementia, early neurologic igns; period sharp wavs on electroencephalogram; absent/rare presence of "florid plaques"; not readily detected in lymphoid tissue
2. vCJD -- death 28 years; median duration 13-14 months; prominent psychiatric/behavioral symptoms, delayed nerological signs; absent sharp eaves on EEC; "pulvinar sign" on MRI; "florid plaques" present in large number; marked acumulation of protease-resistance prion protein in brain tissue; readily detected in lymphoid tissue |
|
|
Which Immunoglobulins cross the placenta?
|
IgG1, IgG2, IgG3, IgG4
|
|
|
Prion diseases share what characterisitcs?
|
1. No latency
2. Long incubation time 3. Gradual increase in severity, leading to death within months 4. No host immune response 5. Non-inflammatory process in brain 6. Neuropathological findings may include: a) normal macroscopic examination b) microscopic spongiform changes, neuronal loss, amyloid plaques |
|
|
How does HPV 16 replicate as an episome? What genes control this process? What do the early genes do?
|
1. main genes that control expression of viral genes is E2--> acts on the viral promoter in way that E6 and E7 expression is expressed at low amount (feedback loop)
a. when the lesion is further progressed- viral genome is disrupted and integrates into cellular genome- i. region disrupted usually in E1 or E2 – becomes linear and integrates ii. integrates at different sites (not at one particular site) --> disrupting E2 gene expression 2. E6 and E7 that is normally controlled by viral promoter--> no more protein (E2) to control efficiency of transcription a. E6 and E7 will be transcribed at high levels (uncontrolled)- act as transforming protein 3. E6 and E7 --> can also immortalize keratinocytes |
|
|
How do prions affect neurons and axons of grey matter?
|
Progressive degeneration.
1. Formation of amyloid containing plaques and fibrils. 2. Proliferation & hypertrophy of astrocytes 3. Vacuolation of neurons |
|
|
What is the immunogloulin involved in mast cell degranulation?
|
IgE
|
|
|
What are the three etilogies involving Prions? What are examples of each? What is the mechanism/explanation of each?
|
1. Inherited -- mutations in PrP gene favoring spontaneous conformational change to ScPrp. Autosomal dominantish. (GSS)
2. Infectious: Exogenous ScPrP inducing conformational change of host CPrP into ScPrP (Kuru, iatrogenic CJD, new variant CJD) 3. Sporadic -- spontaneous conversion (CJD) |
|
|
What do the HPV viruses target? What is the significance of this? What are examples?
|
make complexes with cellular proteins important to regulation of cell growth, proliferation, differentiation and death
i. this inactivates function of cellular proteins ii. ie. HPV E7 complexes with pRb (a normal control of cell cycle) iii. ie. HPV E6- complexes with p53 and BAK iv. only high risk E6 and E7 that can make this association v. oncoproteins of other viruses – (small DNA tumor viruses) -->contain genes that can inactivate pRb and p53 (tumor suppressor genes) 1. ie. adenovirus – E1A and E1B |
|
|
What is PrP? How is it structured? How does it turn infectious? How is conformation indusec?
|
Major (if not only) component of prions.Encoded by endogenous PRNP.
Exists in two conformational isoforms. 1. Cellular (mainly alpha-helices) 2. Scrapie (diseased state) Aberrant metabolism of ScPrP results in accumulation of protein and brain injury. Conformational change of ScPrP is post-translational, induced by presence of other Scs. Chain reaction! |
|
|
What is an idiotope? Idiotype?
|
The unique set of antigenic determinants of the variable portion of an antibody. Each antibody has multiple idiotopes, whose sum gives you the idiotype of the antibody.
|
|
|
What is the molecular and cellular pathogenesis of Prions?
|
1. PrPc tranformed to PrP-Sc
2. Sc accumultion 3. Neuronal death 4. Sc delivered to extracellular compartment 5. Activation of astrocytes & gliosis and neuronal death & apoptosis |
|
|
Activation of a B-cell receptor leads to what possible signaling cascades?
|
Activation of ITAM, or ITIM (inhibitory)
|
|
|
What are some prion-shared clinical features?
|
1. Rapidly progressive dementia
2. Psychiatric symptoms 3. Cerebellar symptoms 4. Involuntary movements 5. Fatality (MK style) |
|
|
What does normal cell cycle progression depend on? How does this work? Where do viral E6 and E7 come into play? What happens when they are expressed and introduced?
|
i. normally cell cycle progression depends on phosporylation state of Rb (when it is phosph it dissociates)
1. Rb binds to transcription factors that are necessary to activate cell factors needed to progress in cell cycle --> and inhibits them a. when viral E7 proteins expressed in the cell --> bind to Rb and dissociates from transcription factors that are now free to activate genes needed for DNA synthesis ii. when viral E6 is expressed it will bind to P53- complex will attach E6-AP (another protein)- acts as a ubiquitin ligand 1. attach ubiquitin residues to p53--> p53 will be targeted to proteosomal degradation 2. unlike with other cancers where the p53 gene is mutated --> in cervical cancer **the gene is normal the problem is the p53 is degraded and inactivated** a. p53 activates genes necessary for activation of apoptosis and inhibition of cell cycle progression |
|
|
What is Kuru? What are the typical syndromes? What are special about this disease?
|
Prevalent in females. Poor communicability. Ataxia, dementia.
|
|
|
What are some of the functions of the Fc receptors?
|
1. Help in movement of AB across a membrane
2. transfer of IgG from mother to fetus across the placenta 3. Engagement of the Ag-AB by the Fc receptor of macrophages and neutrophils help with phagocytosis. |
|
|
How to diagnose prion problems? Which tests are sensitive to what?
|
1. Histopathological examination and immunostaining for PrP-s
2. CSF normal except that mildly elevated protein levels may be seen. Tau protein has highest sensitivity for vCJD, while 14-3-3 good marker for sCDJ. 3. Neuro-imaging test: "hockey tick"and pulvinar sign in vCJD 4. Serial EEG helpful for sCJD, but not vCJD 5. lymphorectuicular tissue good for vCDJ; tonsil biopsy |
|
|
What are the 4 types of protozoa that cause malaria? What is its vector? Where does malaria grow?
|
1. Plasmodium falciparum
2. Plasmodium vivax 3. Plasmodium ovale 4. Plasmodium malariae It is carried by the anopheles mosquito. It grows in the liver and spreads to RBCs. |
|
|
Go through the diagnostic algorithm for prions.
|
Brainstem --> ELISA (+) --> repeat ELISA --> Western immuno-blot --> Described
Brainstem --> ELISA (-) --> Edible wtf this is gross |
|
|
What is IVIG?
|
Intravenous gamma globulin, given to immunodeficient patients. It has a very broad activity.
|
|
|
What is the shape of Rabies? What is its resevoir? Does it depend on location? How is it transmitted?
|
Bullet-shaped, enveloped, helical, -ssRNA.
Resevoir: In US, boats, raccoons, foxes, skunks; worldwide, dogs. Transmission through animal bite. |
|
|
Compare the disease intervals for the malarias.
|
1. Vivax and ovale bursts RBCs every 48 hours.
2. Malariae bursts every 72 hours. 3. Falciparum bursts irregularly b/w 36 to 48 hours. |
|
|
What is the pathogenesis of Rabies?
|
1. Virus binds to peripheral nerves by binding to nicotinic Ach receptor or indirectly into muscle at site of inoculation. It replicates locally at wound for a few days before traveling.
2. Travels by retrograde axoplasmic transport to dorsal root ganglia and spinal cord/ 3. Once it reaches the spinal cord, all hope it lost. It enters the brain and causes fatal encephalitis. |
|
|
What are the two ways anti-idiotypic antibodies function?
|
1. To inactivate antibodies in the serum
2. To inactivate BCRs, via activation of ITIM by crosslinking. |
|
|
What are the clinical symptoms of Rabies? Order them in their respective stages.
|
1. Prodrome -- nonspecific flu-like symptoms. Pain at bite site, minor bacterial/viral-like infection.
2. Acute encephalitis -- confusion, agitation, lethargy, disorientation, hallucination 3. Classical brainstem encephalitis -- hydrophobia 4. Terminal stages -- coma, death |
|
|
What is the life cycle for malaria?
|
1. travels to liver
2. grows, multiplies = sporozides 3. goes back to blood to infects RBCs 4. RBCs harbor merozoites, which will lyse RBCs 5. merozoites can reinfect but also form micro/macrogametrocytes 6. gametocytes drawn outs by mosquito and are fertilized inside 7. penetrate gut, propogate, go to salivary gland |
|
|
How can Rabies be diagnosed?
|
1. Presence of Negri bodies (areas of virus assembly/collections of virions in cytoplasm), intracytoplasmic inclusion bodies.
2. DFA, PCR (post-mortem) |
|
|
What are the different ways the complement system can be activated?
|
1. During Infection
2. In tissue necrosis 3. the coagulation system can activate it. |
|
|
How can Rabies be treated?
|
Once symptoms arise, it can't; however, if it is suspected, then treat with:
1. 1 dose HRIG to bind virus, preventing binding to host cell receptors. 2. 5 doses of killed Rabies vaccine at days 1, 3, 7, 14, 28. |
|
|
How can malaria be treated?
|
1. Malariae, vivax, ovale susceptible to chloroquine; however V & O have exo-eryth. cycles in liver and will be protected there.
2. In non-resistant areas (Central America north of Panama, Hispanola) Falciparum has no exo-erthy, so killed by chloroquine. In resistant areas (Africa), use quinine, artemether. |
|
|
When does the immune system kick in with Rabies? How does it respond?
|
Initially, no response until encephalitis stage. Neutralizing antibodies are produced 1-2 weeks after symptoms or 7-14 days after infection.
Helper and cytotoxic T lymphocyte stimulation and interferon gamma. |
|
|
How can malaria be diagnosed?
|
1. Microscopic examination
2. Rapid antigen based tests |
|
|
From where can Rabies be isolated?
|
1. Saliva
2. Respiratory secretions 3. CSF 4. Tears 5. Brain biopsy |
|
|
The classical pathway starts with what? What part of the immune system is it from?
|
Starts with an antigen-antibody complex. It is part of the adaptive immune response.
|
|
|
How are microbial flora divided? What are some defining characteristics?
|
1. Normal resident flora, if perturbed, prompty reestablishes itself.
2. Transient microbial flora -- may colonize the host for periods ranging from weeks to hours |
|
|
What are pathologies associated with malaria? Which forms cause what diseases?
|
i.renal damage- falciparum + malariae- Ab/Ag complexes deposit in kidney
ii.hemolytic anemia 1.black water fever- sudden onsets of extensive of hemolytic onset --> severe hematuria iii.splenomegaly + hepatomegaly iv.cerebral damage- falciparum v.temperature cycling- when the fever goes down it often goes below normal 1.ovale + vivax - 48 hr cycle of fever bouts 2.malariae – 72 hrs (multiplies slower) 3.falciparum- when it reaches more advanced stages has very uncharacteristic cycles (daily peaks) a.it then begins to resemble 48 hr bouts vi.IgG increases in malaria- higher content of globulin than normal person |
|
|
Where is normal flora located? What are they like? What are examples of each?
|
1. Skin flora --> sparse, gram positive organisms; staphylococcus epidermis, yeast candida albicans
2.Upper respiratory tract flora --> few in lower bronchi and alveolar a.nose --> staph aureus b.throat --> neisseria, staph epidermis c.pharynx --> colonization for neisseria, bordeterlla 3.GI tract flora --> a.mouth --> strep mutans (viridian group) found in large #s in dental plaque the precursor of caries b.stomach --> transient b/c HCl and pesinogen --> low number of H. pylori, lactobacilli, strep d.large intestine --> dense composed predominantly of anaerobes; baceteriods (especially B. fragilis), coliforms, enterococcus, chlostridium 4.Urogenital flora --> often have transient bacteria vaginal --> lactobacillis, s. aureus |
|
|
How does the lectin pathway activate the complement system?
|
Molecules released by the liver, mainly Mannose binding lectin, bind to sugar moieties on the surface of the bacteria and the complement system is activated.
|
|
|
How are mouth flora involved in the formation of cavities? Which bacteria are involved?
|
Strep mutans secrete glucans secreted that form plaque on teeth. Acid produced demineralizes enamel and cause cavities.
Microbial flora found in oral cavivity and streptococcal anaerobes inhabit gingival crevices. |
|
|
How does E5 in HPV work? Where is it located? What is its mechanism?
|
i.E5 small gene downstream from E2 -->when virus is integrated into the host genome this gene wont be expressed (only expressed in early episomal/circular form)
1.contributes to INITIAL states of cancer ii.increases the cell surface EGFR concentration 1.E5 binds to the receptors that are bound to EGF- time the receptor is presented on cell membrane is elongated -->stabilizes receptor that is bound to EGF 2.E5 also binds to an ATPase in the endosome -->inhibits acidification of endosome a.w/o acidification the EGFR is able to be recycled to the membrane- and be activated again |
|
|
What flora are repsonsible for brain, face, and lung infections/abscesse?
|
Anaerobes of oral flora.
|
|
|
How does the alternative pathway activate the complement system?
|
Part of the innate immune response, the alternative pathway activates by the interaction of complement particles with the bacteria itself.
|
|
|
Describe the normal flora in the GI tract? What are the population levels?
|
Stomach – hostile environment for bacteria. Bacterial count highest after meals (103-106).
Helicobacter pylori can survive (peptic ulcer). Jejunum to Ileum – bacterial populations increase may reach levels of 106-108 – Streptococci, lactobacilli, bacteroides and bifidobacteria predominate. Colon – 109-1011 bacteria/g contents. Over 400 species. 95-99% anaerobic genera such as Bacteroides, Bifidobacterium, Eubacterium, Peptostreptococcus and Clostridium. |
|
|
What is indicated on a Pap smear in the presence of cervical cancer?
|
1.koilocytotic cells- cells that contain replicating virus (women who have virus in replicative form before integration into host genome)
|
|
|
What is Clostridium Difficille? Where is it found?
|
Can be part of the normal GI flora but relatively low
Under Ab. Rx. Can stay viable, overgrow and cause disease (pseudomembranous colitis). |
|
|
The smaller peptides cleaved from the main complement proteins, such as C4a, C5a, act as what?
|
They act as anaphylatoxins, and mediate histamine release and mast cell degranulation.
|
|
|
What are anaerobes of the normal flora? Where are they? What are they Are they pathogenic? How is their growth?
|
Bacterium that fails to grow on the surface of solid media in 10% CO2 in air (18% oxygen). Are present only in anaerobic spaces like the intestine.
Are prevalent also in areas exposed to air like skin, nose, mouth. Are not as abundant as aerobic bacteria of the normal flora. Are mainly Gram negative rods. Only a few species can cause infection. Growth and identification are slow. |
|
|
What factors are required for cancer development with EBV? What types of cancers can develop?
|
i.for the development of EBV cancer other factors are required
1.ie. for post transplant lymphomas-->immuno-supression is the co-factor 2.in AfBL cofactor is probably malaria infection 3.in NPC cofactor is probably some dietary/genetic factor Burkitt's lymphoma, Nasopharyngeal carcinoma |
|
|
Are there anaerobes in body areas exposed to air? Why or why not? What is the purpose of this?
|
Also prevalent in body areas exposed to air
Skin: propiobacterium Mouth: peptostreptococci Two explanations: Oxygen consumption by aerobic and facultative flora. Protection from air in microhabitats with low oxidation-reduction potential (gingival crevice, tonsillar crypts and hair follicles). |
|
|
What is the first thing that binds to the changed antibody in the complement activation pathway? What molecules bind after?
|
C1q. This allows for binding of C1r and C1s.
|
|
|
How does normal flora develop? What determines their composition? When do they develop? What affects population?
|
Main factor determining the composition of the normal flora is
pH, temperature, redox potential and oxygen, water and nutrient levels. Other factors: peristalsis, saliva, lysozyme secretion, secretion of immunoglobulins. Infant begins to contact organisms as it moves through the birth canal. Gram-positive population (bifidobacteria and lactobacilli) predominates in early life if infant is breast-fed. Bacterial population is reduced and displaced somewhat by Gram-negative flora (Enterobacteriaceae) when baby is bottle-fed. |
|
|
What are some characteristics of E. coli O157:H7?
|
A.Cause bloody diarrhea
B.Shiga-like toxin is the major virulence factor C.Penetrate the colon epithelium D.Most commonly is acquired from hamburgers E.Does not adhere to brush border |
|
|
What is the purpose of normal flora? How does it achieve this? What are the mechanisms?
|
The normal flora is protective by Bacterial interference
Competing with pathogenic microorganisms for nutrients (interference) Competing for space Competing on receptors (tropism) Producing secreted products that are harmful to some pathogenic microorganisms (bacteriocins/antibiotics). Producing toxic metabolites that are harmful to some pathogenic microorganisms (H2O2, volatile fatty acids, lowering PH) Continued stimulation of the immune system to maintain the immune system primed. |
|
|
C4b and C2b form together to form what enzyme?
|
C3 convertase.
|
|
|
How does lactobacillus engage in bacterial interference? Propionibacterium spp?
|
Lactobacillus colonizes vagina
Lower pH Produce H2O2 Prevent bacterial vaginosis Propionibacterium spp. Hydrolyze triglycerides -> produce FFA, these are inhibitory to S. pyogenes and S. aureus. |
|
|
A florist presents with a subcutaneous lesion on the hand, which she thinks resulted from a jab wound she received while she was making a sphagnum moss-wire frame for a floral wreath. The lesion has not healed despite use of antibacterial cream and has begun to spread up her arm with the lymph node raised and red and beginning to look like it might ulcerate, like the original lesion. The lymph node above is also beginning to redden and is slightly raised. What is most likely to be an appropriate treatment for this infection?
(A) Oral itraconazole or potassium iodide (B) Miconazole cream (C) Cortisone cream (D) Oral griseofulvin (E) Penicillin |
A. This is a classic case of lymphocutaneous sporotrichosis in which a gardener or florist is infected via a puncture wound. The drug of choice is either itraconazole or potassium iodide (administered orally in milk). Topical antifungals are not effective, and the cortisone cream would probably enhance the spread of the disease. Griseofulvin localizes in the keratinized tissues and would not halt the subcutaneous spread of this infection. Penicillin would have no effect because Sporothrix is not a bacterium.
|
|
|
To which digestive processes do normal flora contribute?
|
Vitamin K production (by intestinal bacteria).
Bile formation, regulation of cholesterol metabolism, deconjugation of bile acids (Gram positive anaerobic bacilli). |
|
|
What would you expect to see in the infected gardener's tissue biopsy?
(A) Lots of hyphae (B) Long, branching hyphae with acute angles (C) Yeasts with broad-based buds (D) Cigar-shaped yeasts (E) Yeast with multiple buds (mariner's wheel) |
D. Sporothrix schenckii is dimorphic; the tissue form is cigar-shaped yeasts
|
|
|
When do normal flora cause disease? What are some examples?
|
When normal flora enters sterile sites they should not be in – blood, urine, etc.
GI bacteria moved to urethra -> UTI Skin abrasion/cuts – normal skin flora crosses epithelial cell barrier. URT commensals move to middle ear -> OM |
|
|
C3 convertase does what?
|
Cleaves C3 into C3a and C3b
|
|
|
When can commensals or carried pathogens cause disease? What are some mechanisms?
|
When host-pathogen interaction is modified. Can be modified by:
Host genetic factors Host nutritional status Inoculums Route of infection |
|
|
What are the six damage response curves of the six classes of microbial pathogens?
|
The y axis denotes the amount of damage to the host resulting from the host-pathogen interaction. The x axis denotes the magnitude of the host immune response. Variable refers to the fact that the amount of damage can vary depending on the individual host.
|
|
|
A patient presents with paranasal swelling and bloody exudate from both his eyes and nares, and he is nearly comatose. What is the most likely compromising condition underlying this infection caused by Rhizopus, Mucor, or Absidia (phylum Zygomycota, class Phycomycetes)?
(A) AIDS (B) Diabetes (with patient in ketoacidosis) (C) Neutropenia (D) B-cell defects (E) Chronic sinusitis |
B. Zygomycota are aseptate fungi that cause serious infections, primarily in ketoacidotic diabetic patients and cancer patients. Fungal infections common in AIDS patients include Candida infections (ranging from oral thrush early to fungemias later), cryptococcal meningitis, and disseminated histoplasmosis and coccidioidomycoses. Severely neutropenic patients are most likely to have invasive Aspergillus infections.
|
|
|
C3b-C4b-C2b together form what enzyme?
|
C5 Convertase
|
|
|
What are class 1 pathogens? What are examples?
|
pathogens that cause damage only in situations of weak immune responses.
Legionella pneumophila, Pneumocystis cariini, Psudallescheria boydii |
|
|
What type of fungal skin lesions would show only hyphae and possible arthroconidia in a biopsy?
(A) Blastomycosis (disseminated) (B) Chronic mucocutaneous candidiasis (C) Coccidioidomycosis (D) Dermatophytosis (e.g., tinea pedis) (E) Pityriasis versicolor |
D. Of the choices listed, only the dermatophytes would show hyphae and arthroconidia and cause cutaneous lesions. Blastomycosis (caused by Blastomyces) would show big, broad-based, budding yeasts. Candidiasis would show yeasts, pseudohyphae, and true hyphae. Coccidioidomycosis would show spherules of various sizes with round endospores visible in the large, “mature†spherules. Pityriasis versicolor would have clusters of yeasts with short, septate, curved hyphae (“spaghetti and meatballs†appearance).
|
|
|
What are class 2 pathogens?
|
pathogens that cause damage either in hosts with weak immune responses or in the setting of normal immune responses. In normal hosts, class 2 microorganisms may cause episodic infections, but they do not elicit immune responses that will continue to damage the host after the resolution of an acute infection.
A subset of class 2 is the classical toxigenic bacterial pathogens which damage the host through secreted toxins. In general, protection against toxins is mediated by neutralizing antibody, which binds to the toxin and interferes with toxin-mediated damage. Toxins produce damage rapidly, generally before the immune system can respond. Hence, there is no long-lasting immunity for many toxin-mediated diseases because the amount of toxin produced is presumably not sufficient to stimulate an antibody response. As a result, toxin-producing organisms tend to cause damage irrespective of the immune status of the host, and the damage-response curve for toxigenic pathogens is flat, reflecting the action of the pathogen on the host, in spite of normal immunity. An exception to this generalization is toxigenic Staphylococcus aureus, which produces a superantigen that causes damage by stimulating a T-cell response that can result in the development of toxic shock syndrome. |
|
|
What are class 3 pathogens?
|
pathogens that cause damage in the setting of appropriate immune responses and produce damage at both ends of the continuum of immune responses.
|
|
|
C5 convertase does what?
|
Cleaves C5 into C5a and C5b
|
|
|
What are class 4 pathogens?
|
pathogens that cause damage primarily at the extremes of both weak and strong immune responses.
|
|
|
A 15-year-old dirt biker visiting southern California has pneumonia caused by an organism whose environmental form consists of hyphae that break up into arthroconidia, which become airborne. What is the agent?
(A) Aspergillus fumigatus (B) Blastomyces dermatitidis (C) Coccidioides immitis (D) Histoplasma capsulatum (E) Sporothrix schenckii |
C. Coccidioides immitis is found in desert sand, primarily as arthroconidia and hyphae.
|
|
|
What are class 5 pathogens?
|
pathogens that cause damage across the spectrum of immune responses, but damage can be enhanced by strong immune responses.
|
|
|
What immunoglobulin is considered a better complement activator and why?
|
IgM is better than IgG due to the fact that it’s a pentamer.
|
|
|
What are class 6 pathogens?
|
microorganisms that can cause damage only in conditions of strong immune responses.
|
|
|
Which of the following inhibits ergosterol synthesis, is important in treating Candida fungemias, and is used orally to suppress relapses of cryptococcal meningitis in AIDS patients?
(A) Amphotericin B (B) Fluconazole (C) Griseofulvin (D) Miconazole (E) Nystatin |
B. Fluconazole is an imidazole; all imidazoles inhibit ergosterol synthesis. Fluconazole has become the mainstay in the treatment of serious candidal infections, and it is used to prevent relapse of fungal central nervous system infections in compromised patients. Miconazole, also an imidazole, is available in topical or intravenous preparations and has been largely replaced by the oral fluconazole.
|
|
|
What are examples of class 2 pathogens?
|
|
|
|
Up to what point can the complement cascade proceed without a membrane?
|
Up until the c5 convertase. All the steps before that are in soluble form.
|
|
|
What are examples of class 3 pathogens?
|
|
|
|
A recent immigrant from rural Brazil presents with a swollen face and extremely poor dental hygiene, including loss of an adult tooth, which appears to be the focus of the current infection. There are two open ulcers on the outside of the swollen cheek. Small yellow “grains†are seen in one of the ulcers. Gram stain shows purple-staining fine filaments. What is the most likely disease?
(A) Actinomycotic mycetoma (B) Chromomycosis (C) Eumycotic mycetoma (D) Sporotrichosis (E) Paracoccidioidomycosis |
A. The disease syndrome is lumpy jaw, which is a form of mycetoma. The presenting signs seen in this patient suggest actinomycotic mycetoma, a bacterial infection caused by Actinomyces. (Students: you needed a nonfungal question.) Yeasts will also stain gram-positive. Remember that Actinomyces is a gram-positive anaerobe that is not acid-fast.
|
|
|
What are examples of class 4 pathogens?
|

|
|
|
A normally healthy 8-year-old boy from Florida is visiting friends on a farm in Iowa during the month of July. He presents July 28 with a fever, cough, and lower respiratory symptoms (no upper respiratory tract symptoms). He has been ill for 4 days. His chest sounds are consistent with pneumonia, so a chest radiograph is obtained. The radiograph shows small, patchy infiltrates with hilar adenopathy. His blood smear shows small, nondescript yeast forms inside monocytic cells. What is the most likely causative agent?
(A) Aspergillus fumigatus (B) Blastomyces dermatitidis (C) Coccidioides immitis (D) Histoplasma capsulatum (E) Pneumocystis carinii |
D. Histoplasma and Blastomyces are both endemic in Iowa, but only Histoplasma fits the description of a facultative intracellular parasite circulating in the reticuloendothelial system.
|
|
|
What are examples of class 5 pathogens?
|

|
|
|
After activation of C1s what happens?
|
It cleaves C4 to C4a and C4b, and C4b is covalently bound to the microbial surface.
|
|
|
What is an example of class 6 pathogen?
|
H. Pylori, leading to strong immune responses of gastric gastritis, carcinoma, lymphoma
|
|
|
An uncooperative human immunodeficiency virus (HIV)-positive patient has been complaining of a stiff neck and a severe headache. The headache was initially lessened by analgesics, but the analgesics are no longer effective. His current CD4+ count is 180/mm3. He is not on any prophylactic drugs. What is the most likely causative agent?
(A) Aspergillus (B) Cryptococcus (C) Candida (D) Malassezia (E) Sporothrix |
B. Cryptococcus, an encapsulated yeast, is the major causative agent of meningitis in patients with AIDS.
|
|
|
What are PBPs? How do they work? What do they contain? Where are they made? How do they vary?
|
Penicillin-binding proteins. Bacteria produce four types of PBPs (1-4).
PBPs differ in their affinities for binding ß-lactam antibiotics, which explains why ß-lactam antibiotics differ in their antibacterial properties and spectrum of activity. Affects the morphologic response PBPs transmit a transmembrane signal for induction of ß -lactamases. ß -Lactamases are PBPs that catalyze hydrolysis of the ß -lactam ring, thereby inactivating the drug. The enzymes are different in Gram-positive and Gram-negative bacteria and in anaerobic species |
|
|
What happens next?
|
C4b binds C2, which is cleaved by C1s into C2a and C2b. Forming the C4b2b complex.(C3 convertase)
|
|
|
What is the autolytic system? How does this affect antibiotics? What is an example of an autolytic enzume? Where is it found?
|
Beta-lactam inhibition of cell wall synthesis leads to activation of the autolytic system through a two component system, VncR/S, which initiates a cell death program
Certain bacteria are deficient in autolytic enzymes or have mutations in the regulatory genes; these strains show the phenomenon of "tolerance" to beta-lactam antibiotics: their growth is inhibited by the antibiotic but the bacteria are not killed In PCN, murein hyrdolases, which degrade PDG. |
|
|
A logger undergoing chemotherapy for cancer has developed pneumonia and skin lesions. Biopsy of the skin lesions demonstrates the presence of large yeasts with thick cell walls and broad-based buds. What is the most likely causative agent?
(A) Aspergillus fumigatus (B) Blastomyces dermatitidis (C) Coccidioides immitis (D) Histoplasma capsulatum (E) Sporothrix schenckii |
B. Blastomyces has a double refractile wall and buds with a broad base of attachment to the mother cell. The environmental association appears to be rotting wood.
|
|
|
What are examples of inhibitors of cytoplasmic membrane?
|
Antibacterials: polymyxins
Antifungals: Polyenes: amphotericin B Azoles: fluconazole, itraconazole, voriconazole, posaconazole |
|
|
Which enzyme initializes the lytic cascade, and where is it found.
|
C5 convertase starts the lytic process, It is bound to the microbial membrane.
|
|
|
What are Polymyxin B and colistin (polymyxin E)? How do they work? Which bacteria do they affect? How are they used?
|
Bactericidal, cyclic polypeptides
Free amino groups act as cationic detergents disrupting the phospholipid structure, leak, death Inhibit Gram-negative bacteria Bind to various ligands in body tissues and are potent toxins for the kidney and nervous system Used mainly topically, now very popular |
|
|
About how many C9s are required to form the pore?
|
About 15
|
|
|
How do inhibitors of fungal cytoplasmic membrane work?
|
Fungal membranes contain sterols, bacterial membranes do not
Polyene bind to membrane sterols, make a pore in the fungal membrane and the contents of the fungus leak out Azoles Inhibit ergosterol synthesis |
|
|
Which of the following is the most frequent cause of blood transfusion–associated hepatitis?
(A) Hepatitis A virus (B) Hepatitis B virus (C) Hepatitis C virus (D) Hepatitis D virus |
C. Hepatitis can be transmitted by both the oral and parenteral routes; hepatitis C virus is the virus most associated with hepatitis following the transfusion of blood products.
|
|
|
What are examples of inhibitors of NA synth?
|
Inhibitors of DNA replication:
quinolones nitroimidazoles Inhibitors of RNA polymerase: rifamycins Inhibitors of nucleotide metabolism: acyclovir, AZT (viruses) flucytosine (fungi) |
|
|
What is the trigger of the alternative pathway?
|
The binding of C3 to the surfaces of certain pathogens. C3b binds to the surface of the bacterium.
|
|
|
Anti–viral capsid antigen (VCA) antibodies are found in
(A) cytomegalovirus infections. (B) Epstein-Barr virus infections. (C) herpes simplex virus infections. (D) varicella-zoster virus infections |
B. Antibodies to the viral capsid antigen (VCA) are important in identifying Epstein-Barr virus infections.
|
|
|
How do quinolones work? What do they do?
|
Block the action of the bacterial enzymes DNA topoisomerases: DNA gyrase & topoisomerase IV
DNA gyrase produces and removes supercoils in DNA ahead of the replication fork to maintain the proper 'tension' required for efficient DNA duplication Topoisomerase IV acts to remove catenanes |
|
|
What is the mechanism of quinolone function?
|
Block bacterial topoisomerases by binding to the complex of DNA gyrase and DNA
DNA gyrase is the primary target for Gram-negative bacteria Topoisomerase IV is the primary target for Gram-positive bacteria. Topoisomerase IV comprises two subunits, ParC and ParE. The ParC protein is homologous to the gyrase A protein, while the ParE subunit is homologous to the gyrase B protein. The other enzyme being the secondary target |
|
|
What does factor B do?
|
Attaches to the C3b to form C3b-Bb, which functions as a C3 convertase.
|
|
|
What are Nitroimidazoles?
|
The nitro group is reduced by anaerobic bacteria
Reduced products cause strand breaks in DNA Mammalian cells are unharmed because they lack enzymes to reduce the nitro group of these agents |
|
|
Passive immunization is available for protection from
(A) influenza A virus. (B) hepatitis A virus. (C) parainfluenza type 2 virus. (D) rubella virus. |
B. A commercially available human immune globulin preparation is available for pre- and postexposure prophylaxis for hepatitis A virus.
|
|
|
How do Rifamycins work?
|
Binds to a beta-subunit of RNA polymerase, blocks the synthesis of mRNA
Selective toxicity is based on greater affinity for bacterial polymerases than for human enzymes |
|
|
What cleaves Factor B?
|
Factor D
|
|
|
How does fluctosine work?
|
Interfere with purine and pyrimidine synthesis
Inhibits yeasts Converted in the fungal cell to 5-fluorouracil, which inhibits thymidylate synthetase resulting in a deficit of thymine nucleotides and impaired DNA synthesis |
|
|
Which of the following is the first viral-induced defense mechanism in a nonimmune individual?
(A) Generation of cytotoxic T lymphocytes (B) Production of interferon (C) Synthesis of lymphokines (D) Synthesis of neutralizing antibodies |
B. The production of interferons that induce the synthesis of antiviral replication proteins in neighboring cells occurs before the appearance of any other viral-induced immune defense mechanisms.
|
|
|
What are examples of inhibitors of ribosome function? Differentiate b/w which ribosomal subunits they affect.
|
Inhibitors of 30S subunits
Aminoglycosides (Irreversible) Tetracyclines (reversible) Inhibitors of 50S subunits Macrolides Ketolides Lincosamides Streptogramins Chloramphenicol Oxazolidinones Fusidic acid |
|
|
What is the function of Factor P or properdin?
|
It stabilizes the C3b-Bb or C3 convertase in the alternative pathway.
|
|
|
How do tetracyclines work?
|
Bind to 30S ribosomes
Inhibit the binding of aminoacyl-tRNA into the A site of the bacterial ribosome Tetracycline binding is transient, bacteriostatic The selective action is due to their greater uptake by prokaryotic cells |
|
|
Where are viral oncogenes found?
(A) JC virus (B) Human T-cell lymphotrophic virus type I (C) Rous sarcoma virus (D) Simian virus 40 |
C. Viral oncogenes are found in many RNA tumor viruses. Both Rous sarcoma virus and human T-cell lymphotrophic virus type I are RNA tumor viruses, but only Rous sarcoma virus carries an oncogene (v-src).
|
|
|
What is the function of C3a?
|
It leads to increased vascular permeability and activation of phagocytosis of the microorganisms by PMNs and macrophages.
|
|
|
How to aminoglycosides work?
|
Bactericidic
AG bind to specific proteins (S12) in the 30S ribosomal subunit, interfere with the binding of met-tRNA to the ribosome, preventing the formation of initiation complexes AG cause misreading of mRNA codons |
|
|
Which bacteria are competent? What does that mean? What is this involved in? What is the problem?
|
Transformation is a mode to introduce naked DNA into bacteria.
Streptococcus pneumoniae, Haemophilus influenzae, Neisseria gonorrhoeae are naturally 'competent' to take up DNA fragments from related species across their cell walls Most bacteria are not naturally competent to be transformed by DNA Prior to uptake by competent cells, DNA is vulnerable to destruction. it is the least important mechanism of gene transfer |
|
|
Which of the following is a viral protein that is thought to induce tumors by binding to a cellular tumor suppressor protein?
(A) Adenovirus E1A (B) Epstein-Barr nuclear antigen proteins (C) Hepatitis B virus e protein (D) Human immunodeficiency virus gag protein |
A. In permissive cells, adenovirus E1A protein is involved in the replication process, but in nonpermissive cells it can bind to cellular tumor suppressor protein p110Rb and inactivate its normal cellular function, which results in cellular transformation.
|
|
|
What is transduction? How does it work? How is it carried out?
|
Introduction of DNA by bacteriophage
Transducted DNA is always protected, thus increasing its probability of successful transfer and potential clinical relevance Bacteriophages are extremely host-specific 'parasites', unable to move any DNA between bacteria of different species |
|
|
What is the function of C5a?
|
It is chemotactic for neutrophils, increases vascular permeability, releases histamine from mast cells.
|
|
|
What is conjugation? What does it depend on? Is it efficient?
|
Introduction of DNA by cell to cell contact, bacterial ‘mating’
Dependent upon tra genes which found in 'conjugative' plasmids, encode sex pilus which allows cell-to-cell contact and plasmid DNA transfer Rapid and highly efficient movement of genetic information through bacterial populations, highest clinical relevance |
|
|
What is unaffected by aminoglycosides? What is especially sensitive?
|
Eukaryotic ribosome in the cytosol is relatively unaffected by these drugs,
Ribosomes in the mitochondria are 70S and sensitive to their effects |
|
|
What does CR1 bind and what is its function?
|
It binds C3b and C4b, and it functions to cause opsonization.
|
|
|
What are examples of resistances of chromosomal origin? Whare are examples? Which bacteria are resistant to what drugs? How common is this?
|
Point mutations: a single chromosomal mutation resulting in the synthesis of an altered protein: streptomycin resistance - S12, single amino acid change in dihydropteroate synthetase - lowered affinity for sulfonamides
Rearrangements: inversions, duplications, insertions, deletions, or transposition of large sequences of DNA from one location of a bacterial chromosome to another, created by transposons or insertion sequences: changes in penicillin-binding proteins (PBPs) in penicillin-resistant pneumococci This is relatively rare. |
|
|
How does Chloramphenicol work?
|
Inhibits peptide bond formation by binding to peptidyltransferase on the 50S ribosome
Although mammalian cells contain primarily 80S ribosomes that are unaffected by chloramphenicol, the mitochondria do contain 70S particles |
|
|
How do the erythrocytes function to reduce kidney inflammation during severe infection?
|
They bind C3b via the CR1 receptor, and hand the molecules off to a macrophage, preventing accumulation in the kidneys.
|
|
|
What is the most important type of gained resistance? Is it stable? how does it work? What are examples? What are some characteristics? What is it associated with?
|
Transmissible (TEM-1 b lactamase spread from the Enterobacteriaceae to H. influenza and N. gonorrhoeae)
Highly stable Confers resistance to many different classes of antibiotics simultaneously Antimicrobial agents exert a strong selective pressure Associated with other characteristics that enable a microorganism to colonize and invade a susceptible host |
|
|
How do Oxazolidinones (Linezolid) work?
|
Bind to the 50S ribosome at a site near the 30S ribosome interface, prevent the 30S initiation complex from forming the 70S initiation complex. The initiation of protein synthesis is blocked
|
|
|
Which complement proteins mediate aggregation of platelets?
|
C3a and C5a
|
|
|
What are the biochemical mechanisms of antibiotic resistance?
|
Drug inactivation
Reduced target affinity Reduced permiability Antibiotic Efflux Bypass of Antibiotic |
|
|
How does fusidic acid work?
|
Forming a stable complex with elongation factor EF-G (the bacterial equivalent of the human EF-2), guanosine diphosphate and the ribosome
|
|
|
What does DAF do?
|
Binds to the C3 convertase, releases factor Bb, and it enables Factor I to cleave C3b and inactivate it.
|
|
|
What are examples of drug inactivation? Which is the most important? How does it work? What is affected?
|
1. beta-lactamase (most important)
2. aminoglycoside modifying enzymes 3. chloramphenicol acetyltransferase 4. erythromycin Enzymes that inactivate b-lactams by splitting the amide bond of the b-lactam ring. May cleave predominantly penicillins (penicillinases), cephalosporins (cephalosporinases), both (beta-lactamases), ESBL |
|
|
How is folic acid useful in terms of creating antibiotics? What are inhibitors?
|
Bacteria and human hosts require folic acid for
(a) nucleic acid synthesis (b) protein synthesis (precursor of the amino acids methionine and glycin) Humans ingest folic acid already formed (folate= vitamin) Bacteria lack a transport system to take up preformed folic acid from their environment. Bacteria must synthesize folates, starting with para-aminobenzoic acid (PABA) Sulphonamides |
|
|
What prevents the function of C5b?
|
CD59.
|
|
|
Where are beta-lactamases excreted in gram pos and meg bacterias? What are the mechanisms of each? What are problems?
|
In Gram positive bacteria b-lactamases are excreted into the environment, in Gram negative b-lactamases are excreted in the periplasm.
β-lactamase is the in the periplasm for the gram negative and released into the environment for the gram positives. This is good for the bacteria because there is a high concentration of the β-lactamase before the antibiotics come to the targets which are the PBP. So, you can see that in gram negatives, it is very difficult to act. In gram positive, the β-lactam comes and diffuses through the peptidoglycan and binds directly to the PBP. On the other hand, in gram negatives, it is very difficult for the β-lactam to cross the outer membrane so some β-lactam can cross the membrane through the porin channels but if the β-lactams cross the porin and come to their targets, then there is a high concentration of the β-lactamase and it will destroy the β-lactam. So, it is much harder to get destroy the β-lactam in gram negative than in gram positive. |
|
|
What is the difference b/w bactericidal and bacteriostatic antibiotics? Which is more effective?
|
Bactericidal: killing the target bacterium or fungus
Bacteriostatic: inhibiting its growth Bactericidal agents are more effective, but bacteriostatic agents can be extremely beneficial since they permit the normal defenses of the host to destroy the microorganisms |
|
|
Which cells secrete complement?
|
Major source is the liver, but macrophages can too.
|
|
|
What is the difference between inducible and constitutive beta-lactamases? What are examples of inducers?
|
Constitutive: constant level of production (as for many plasmid-mediated enzymes)
Inducible: level of production varies with the presence of antibiotic (as for many chromosomal enzymes) Strong inducers: imipenem, ampicillin, cefoxitin, clavulanate Moderate inducers: sulbactam Weak inducers: ceftriaxone, ureidopenicillins, tazobactam |
|
|
How to choose b/w bactericidal and bacteriostatic agents?
|
Type of infection: cystitis: bacteriostatic antibiotics. Endocarditis: bactericidal antibiotics. Invasive bacterial infection such as meningitis, osteomyelitis are best treated with bactericidal agents
Host factors: neutropenic patients, best treated with bactericidal drugs Type of organism: enterococci require two agents for killing |
|
|
How can beta-lactamases be mediated? How are they made/acquired? What are these characteristics of?
|
Plasmid or chromosomally mediated.
b-lactamases production may be encoded within the bacterial chromosome (characteristic of an entire species) or genes may be acquired on a plasmid or transposon (characteristic of an individual strain). |
|
|
Which antibiotics are bacteriostatic? Bactericidic?
|
1. Bacteriostatic tetracycline sulfonamides clindamycin chloramphenicol
2. Bactericidic beta-Lactams aminoglycosides fluoroquinolones glycopeptides rifamycin |
|
|
What are the different strains of b-lactamases? What do they account for? What are examples? To where are they endemic?
|
TEM-1:
Accounts for 75 to 80% of plasmid-mediated ß-lactamase resistance worldwide Mainly in H. influenza, E. coli, Salmonella, Shigella, N. gonorrhoeae ESBL (TEM 3,5,8,12,24…): Extended Spectrum Beta Lactamases, confer resistance to all penicillins and cephalosporines. Endemic problem in many hospitals |
|
|
What is transformation? Which bacteria engage in this? How important is this uptake method?
|
Introduction of naked DNA
Streptococcus pneumoniae, Haemophilus influenzae, Neisseria gonorrhoeae are naturally 'competent' to take up DNA fragments from related species across their cell walls Most bacteria are not naturally competent to be transformed by DNA Prior to uptake by competent cells, DNA is vulnerable to destruction. it is the least important mechanism of gene transfer |
|
|
What are the four molcular classes of b-lactamases? How do they work? What do they include? What is at their active sites? Are they inducible?
|
A: Serine at the active site, encoded mostly on plasmids, constitutively expressed, preferential hydrolysis of penicillins. Include: TEM-1, SHV-1, penicillinase in S. aureus
B: Metalloenzymes, zinc atom at the active site, preferential hydrolysis of carbapenems. Include: IMP-1 C: Serine at the active site, determined by the chromosomal ampC gene, inducible, mainly cephalosporinase. Include: MIR-1 D: Serine at the active site, hydrolyze Oxacillin, OXA-1 |
|
|
What are 4 mechanisms mediating bacterial reistance to drugs?
|
a.bacteria produce enzymes that inactive the drug
i.ie. β lactimases inhibit PCN and cephalosporines b.bacteria synthesize modified targets in which drug has no effect i.ie. mutant protein in 30S subunit --> resistant to streptomycin c.bacteria decrease permeability such that effective intracellular concentration of drug cannot be achieved i.changes in porins reduce amount of PCN entering bacteria d.bacteria actively export drugs using an MDR pump i.MDR pump imports protons in exchange for variety of foreign molecules (ie. quinolone antibiotics) |
|
|
What are aminoglycoside modifying enzymes? How are they coded? What are the reactions involved?
|
>24 aminoglycoside-modifying enzymes
Coded by genes on plasmids or the chromosome Three reactions: N-acetylation (AAC), O-nucleotidylation (ANT), O-phosphorylation (APH) |
|
|
What is chromosome mediated resistance?
|
Due to a mutation in gene that codes for either target of drug or transport system in membrane controlling uptake of drug. In the presence of antibiotic, these spontaneous mutants have a selective advantage to survive and outgrow the susceptible population.
|
|
|
How do Chloramphenicol acetyltransferase and Erythromycin esterase work?
|
It reduces target affinity through:
Alteration of target enzymes Alteration of ribosomal target sites Alteration of cell wall precursor targets |
|
|
Why is plasmid-mediated resistance important?
|
1.it occurs in many species (especially gram – rods)
2.plasmids frequently mediate resistance to multiple drugs 3.plasmids have high rate of transfer from one cell to another (via conjugation) |
|
|
What are examples of alteration of target enzyme? How do they work? What are examples of each?
|
1. Altered PBP (transpeptidases, carboxypeptidases): confer decreased affinity for b Lactams
Methcillin resistance Staphylococcus aureus (MRSA) and Staphylococcus epidermidis (MRSE): A new PBP is formed, PBP2a encoded by mecA gene. PBP2a: capable of cell wall synthesis in the absence of other PBPs, has low affinity for all β-lactams (and also to methicillin) Penicillin resistance in Streptococci pneumoniae (PRSP): decreased affinity of some PBPs, loss of others, new PBPs 2. Altered synthetase: resistance to sulfonamides 3. Altered DNA gyrase: Resistance to quinolones Chromosomal mutations Gram negative mainly in gyrA locus, Gram positive in parC |
|
|
What are characteristics of plasmid-mediated resistance? Is it transmissible? Stable?
|
Transmissible (TEM-1 lactamase spread from the Enterobacteriaceae to H. influenza and N. gonorrhoeae)
Highly stable Confers resistance to many different classes of antibiotics simultaneously Antimicrobial agents exert a strong selective pressure Associated with other characteristics that enable a microorganism to colonize and invade a susceptible host |
|
|
What are examples of resistance mechanisms that utilize alterations of ribosomal target sites? How do they work?
|
1. MLS (Macrolide,Lincosamides, Streptogramin) resistance
Acquisition of erythromycin resistant methylase (erm) genes that encode 8 classes of enzymes that methylate adenine residue on 23S rRNA of the 50S subunit Conformational changes in the ribosome, results in decreased affinity and leads to co- resistance to all MLS antibiotics Plasmid or chromosomal encoded 2. Aminnoglycoside resistance: mutation of S12 protein of 30S subunit |
|
|
What are the different ways S. Aureus be treated? What happened? Why is this important? What are the different types of resistant staph? What is so problematic now?
|
1. Penicillin-resistance: S. aureus developed beta-lactamase, giving ability to destroy the beta-lactam (Penicillin).
2. Methicillin-resistance: Meth has huge side chain that masks the site of the beta-lactam. Beta-lactamase cannot cause its damage to the beta-lactam ring; however, Aureus developed a new mechanism of resistance, changing penicillin binding proteins. Point mutation in the PBP so penicillin can no longer bind to its target. (MERSA) 3. Vancomysin: Toxic, very expensive and might cause even more resistant bacteria. We have enterococci. Usually enterococci are susceptible to very simple antibiotics. But now we are using vancomysin. There was a fear that our simple enterococci would become resistant to vancomysin (VRE). Because we use a lot of vancomysin, the enterococci in our stool became resistant to vancomysin. If these resistant enterocci share their resistance with staph aureus we might get this super bug that is also resistant to vancomysin…and this is a problem because we will not know what to give the patients. In 1997, we found the first Staph Aureus isolate with intermediate vancomysin resistance. It is not completely resistant. We call this bug VISA. Vancomysin is not active against VISA and we therefore have a lot of problems. Many antibiotics are under clinical study but this is still a real problem. Since 2002 we have found 5 isolates of vancomysin resistant bacteria (VERSA) which are fully resistant to vancomysin. Another very important issue is that (mainly in the States) these resistant bacteria are no longer confined to the hospitals; have cases of cMERSA (community-acquired MERSA). |
|
|
What is a successful and effective vaccine?
|
Good protective antigens; represent natural viral antigen, accessible for the immune system components.
It must be safe Must produce protective immunity Must generate long-lived immunological memory Must be cheap |
|
|
What are major sites in viral infection? What are examples?
|
1. Infection via mucosal surfaces: respiratory tract and gastro-intestinal tract. corona; parainfluenza; respiratory syncytial; rota
2. Infection via mucosal surfaces, systemical spread via the blood and/or neurons to target organs. picorna; hepatitis A and E 3. Infection via needles or insect bites, followed by spread to target organs. hepatitis B; hepatitis C; flaviviruses |
|
|
What is an example of resistance acquired through alternation of cell wall precursors? What is its mechanism?
|
Glycopeptide resistance (vancomycin, teicoplanin)
Glycopeptides bind to D-alanyl-D-alanine at the termini of peptidoglycan and prevent transglycolysation and transpeptidation The genes associated with glycopeptide resistance in enterococci: vanA, vanB, vanC, vanD which encode a ligase producing pentapeptides terminating in D-alanyl-D-lactate = depsipeptide |
|
|
What are problems in the development of vaccines?
|
1. Antigenic drift and shift
2. Large animal reservoirs - Reinfection may occur 3. Integration of viral genome- Vaccination against latent viruses are problematic, HSV 4. Transmission from cell to cell via direct cell-cell contacts (syncytia) 5. Recombination of the virulent strain or of the vaccine virus (polio virus) |
|
|
Is there emergence of glycopeptide-resistance enterococci?
|
United States: prolonged or indiscriminate nosocomial use of vancomycin, by either the oral or the intravenous route
Europe: use of avoparcin, a glycopeptide-like substance as an animal feed supplement |
|
|
Is there an animal reservoir for variola? How frequent are subclinical cases? Describe its infectivity. How many serotypes exist?
|
There is no animal reservoir for variola
Subclinical cases rare - infected person can be identified and isolated Infectivity does not precede overt symptoms, that is there is no prodromal phase There is only one Variola serotype - the vaccine effective against all virus strains Lifelong immunity |
|
|
How is resistance acquired through reduced permeability?
|
Outer membrane (OM) acts as a barrier to the penetration of antibiotics into the cell
OM is absent in gram-positive bacteria |
|
|
What was the first attenuated vaccine developed? What do we use now?
|
Rabies. We now use an inactivated virus.
|
|
|
What are porins? How are they used? Where are they produced?
|
Proteins that are arranged to form water-filled diffusion channels through which antibiotics may traverse
Bacteria produce a large number of porins (105 porin molecules in a single cell of E. coli) Bacteria are able to regulate the relative number of different porins in response to the osmolarity of the surrounding media In hyperosmolar media, E. coli may repress production of the larger porins (OmpF) while continuing to express smaller ones (OmpC) |
|
|
Name some vaccines that use live virus.
|
1. Influenza A & B
2. MMR 3. Polio 4. Rota 5. Varicella, Zoster 6. Adeno 7. Smallpox 8. Yellow Fever |
|
|
Describe the diffusion of antibiotics through OM. How is it facilitated?
|
Now the diffusion of the antibiotics through the outer membrane are really influenced by the numbers and properties of the porin channels. If we have many porins and many big porins, the antibioics can diffuse very easily. On the other hand, if there are not as many large porins, there is less diffusion of antibiotics. Another important issue is the characteristics of the antibiotics. For example, if there is a large antibiotic molecule, it will not cross the porin. If it has more negative charges it will be more difficult to cross through the porins. The greater the degree of hydrophobicity, the less likely it will penetrate through the porin. Small hydrophilic molecules with a zwitterionic charge, such as imipenem, are highly permeable.
|
|
|
Which vaccines use killed virus?
|
1. Hep A
2. Flu A & B 3. Polio 4. Rabies |
|
|
When do we have reduced permeability in bacteria membrane? Does this present a problem? How is resistance created? What is an example?
|
When do we have reduced permeability? We don’t have reduced permeability in gram positive bacteria because in gram positive bacteria we do not have an outer membrane. This is only a problem in gram negative bacteria. We have a drug called imipenem which is very active. We show that every bug is susceptible to this antibiotic especially the P. aeruginosa which is a very problematic and resistant bug. The P. aeruginosa became resistant to the imipenem due to a loss of the D2 porin. The P. aeruginosa has a specific porin for the imipenem, the D2 porin and when the imipenem was introduced to the P. aeruginosa what it did was that the D2 porin disappeared. The P. aeruginosa was able to close the porin. So, this is a very important mechanism of resistance in P. aeruginosa. This kind of resistance can be to many kinds of antibiotics including beta-lactams, aminoglycosides, and quinolones.
|
|
|
How are attenuated vaccines made? What are the advantages of using them? Disadvantages?
|
Growth in non-optimal condition to select for mutants that cannot replicate well in the human body -- growth in nonhuman hosts --> growth in tissue culture --> growth at lower temperature selecting for cold adapted mutants
Advantages: 1. mimic natural infection 2. activate humoral and CMI responses 3. provides LOCAL immunity (IgA) 4. balanced immunity to all antigens 5. more long lasting immunity + more cross reactive (stimulate Ab against multiple epitopes which are similar to those elicited by the wild type virus) 6. low cost Disadvantages: 1. reversion to virulence (polio by back mutation) 2. contamination with other agents (polio SV40) 3. residual virulence --> a. CI: immune suppresses + pregnant 4. persistent infection (rubella) 5. interference by co-infection with a naturally wild-type virus 6. storage conditions (needs to be kept in cold) and limited shelf life 7. more problems giving vaccine in areas where there isn’t proper cooling (Africa) |
|
|
How is resistance acquired through antibiotic efflux? What are the mechanisms? Where are they found?
|
Active efflux of antibiotic by inner membrane protein
Major mechanism of resistance in Gram negative bacteria to tetracycline, mef gene resistance to macrolides, Quinolones Found among E. coli, Shigella, Pseudomonas |
|
|
What are the advantages and disadvantages of using inactivated vaccine?
|
Advantages: no risk of virulence
Disadvantage: risk of incomplete inactivation 1. need repeated administration- usually “3 dose” vaccination 2. limited local immunity (low IgA) 3. poor CMI (antigens not presented by natural way) |
|
|
How is resistance acquired through bypass of antibiotic?
|
Folate auxotrophs: able to use pre-formed folates, exhibit reduced susceptabilites to sulfonamides and trimethoprim
Overproduction of DHFR: trimethoprim resistance |
|
|
What are 4 anti-viral strategies? Describe each.
|
a. Virucidal Agents;
i. Direct inactivation- detergents (topical use- rabies; nonoxynol-9 in birth control jellies inactivates HSV, HIV) b. Immunostimulatory drugs: i. immunization: passive (rabies antibodies), active ii. interferon inducers iii. agents augmenting CMI c. Anti-viral agents i. inhibition of intracellular viral replication ii. why use anti-virals: 1. for treating immunosupressed pts 2. for viruses w/ no-effective vaccine, multiplicity of serotypes or constant mutations d. difficulty in identifying a safe drug that can be effective--> b/c viruses are obligate intracellular parasites i. discovery- screening or rational design ii. best antiviral agent- inhibit specific step in viral replication |
|
|
What are the three theories concerning the origin of viruses? Not including the clearly correct one about viruses being aliens from outer space.
|
a. regressive evolution- viruses degenerated from previously independent life forms that lost many functions that other organism have and retained only want is needed for their parasitic life cycle
b. cellular origins- comes from subcellular component of macromolecules that have gained capacity to move from cell to cell c. independent entities- evolved on parallel course to cellular organisms |
|
|
Describe the inactivated polio virus. What does it do? What are problems?
|
A formalin-killed preparation of normal wild type polio virus
Given by injection (IPV) Elicits good humoral (IgG) immunity and prevents transport of the virus to the neurons where it would otherwise cause paralytic polio. Problems - little local immunity, wild type can replicate in respiratory tract, gastro-intestinal tract virus is shed in the feces (no elimination). |
|
|
What are the selective forces that drive virus evolution?
|
The sources of this diversity are mutation, recombination, and selection. The selective forces working on viruses are imposed by the environment or the constrains of the viral genome and life cycle.
|
|
|
Describe the live polio virus. How is it produced and given? What does it do? What is the difference b/w the Sabin vaccine and the Salk vaccine?
|
Produced by serial passage in cell culture
selection of a mutated virus that grew well in kidney cell culture in the human gut It is given orally (produces a local immune response in intestine lining- poliovirus multiplication site) Gives gut immunity via IgA elicits humoral and cell-mediated immunity It cannot migrate to the neurons only one administration is needed immunity last long The Sabin vaccine-a potential to wipe out wt-virus The Salk vaccine only stops the wt-virus getting to the neurons - still replicated in the human gut |
|
|
What is the difference b/w RNA and DNA viruses in terms of mutant genomes?
|
RNA viruses are copied with considerably less fidelity, error rates are high.
DNA viruses tend to have low error rates during genome replication because of error-correcting DNA polymerases . Error rates are somewhat higher than that observed for the host genomes. DNA viruses that establish latent infections do not replicate their genomes extensively. As a result, diversity is reduced. Herpesviral genomes that establish long lived latent infection change at about the same rate as that of the host. |
|
|
What is the problem with the Sabin (OPV) vaccine?
|
Back mutation: may result from recombination between wild type virus and the vaccine strain (1:4M)
contaminating pathogens (SV40 ) |
|
|
What are the constraints for the stability of the virus?
|
i. genome size limit (ie icosahedral capsids have defined internal space)
ii. proteins required for viral replication and interactions with cellular components |
|
|
What is a subunit vaccine? What is it used to treat?
|
New approach for inactivated vaccine.
Purification of natural antigens- subunit vaccine 1. purify component of virions --> a. HPV contains viral major capsid protein b. HBV- surface antigens c. Influenza – take only HA and NA from virus and use as the vaccine |
|
|
What are two general pathways for viral revolution?
|
Viruses coevolve with their hosts so that both share a common fate;
as the host prospers so does the virus. loss of the host results in loss of the virus. DNA viruses tend to follow this pathway. Viruses have multiple host species. When one is compromised, the virus can replicate in another. RNA viruses tend to follow this pathway. |
|
|
What is a recombinant viral antigen?
|
A viral protein (surface glycoprotein) expressed in cell culture purified and injected intramuscularly.
- Bacteria -lack of post-translational processing - Yeast (process glycoproteins) - Mammals cells (CHO) |
|
|
What is the definition for an "emerging virus?"
|
newly appeared in the population or is rapidly expanding its range of hosts with a corresponding increase in detectable disease
|
|
|
What are the three sources of viruses?
|
1. Mutant virus already existing in an infected host can be selected
2. A virus can enter a naïve population from an infected population of the same species 3. A virus can enter a naïve population from an entirely different infected species |
|
|
What are adjuvants?
|
are nonspecific stimulators of the immune response. When mixed with an antigen, help to deposit the injected material helping to increase antibody response.
|
|
|
What HBV vaccines exist? What do they do? How do they work? What are their limitations?
|
ENGERIX-B
Derived from hepatitis B surface antigen (HBsAg) Produced in yeast cells (Saccharomyces cerevisiae cells). RECOMBIVAX HB Contain the middle and small envelope proteins (HBsAg and PreS2) Transfected in Chinese hamster ovary cells. Limitations: High cost for (problem -endemic area) at least three injections are requirement to achieve protection Small number of individuals do not develop protective immunity Escape variants with mutation within HBsAg |
|
|
What are examples of emerging viruses? (We need to know these/have already learned these)
|
1. Flu
2. Denuge 3. Sin Nombre 4. Hanta Virus 5. Ebola 6. HIV 7. HTLV 8. Norwalk |
|
|
What are VLP? How are they used? What vaccines are being developed?
|
It is expected that cervical cancer can be prevented by prophylactic vaccination that reduces the burden of infection in a population
Vaccination against HPV infection using genotype-specific HPV L1 VLPs. Two vaccines currently developed: Gardasil® –HPV types 16, 18, 6 &11 Cervarix ® –HPV types 16 and 18. Efficacy demonstrated for up to 5.5 years after vaccination. Requirement for booster dose at later time not established. Routine cytology screening still required, as vaccines do not protect against all oncogenic types of HPV. |
|
|
In therms of WEE, EEE, what are dead end hosts? What is host I? Host II? What is the vector?
|
Humans and horses.
I -- wild birds II -- chickens mosquitos |
|
|
What are synthetic peptides? How can they be used in vaccine production?
|
Prediction of viral antigenic regions
linear sequence might not mimic the native conformation of the parent antigen Raise an IgG- Poor antigenicity – strong adjuvants needed: Liposomes Integrate peptides by genetic engineering into carrier proteins within a viral vector Chemically well defined, selective and safe. Stable at ambient temperature. Useful in inducing the generation of T cells: T cell epitopes- linear peptides associated with MHC haplotype |
|
|
What is the infectious cycle of the european tick-borne encephalitic?
|
Tick infects rodent, which infects tick, which can either infect a human directly or a goat (and consequently human through its milk)
|
|
|
In which diseases do rodents play critical roles in the introduction of new viruses?
|
Hemorrhagic disease viruses
Lassa – W.Af (f,p,e,h) Junin, Macupo-S.Am arenaviridae Hantaviruses – Bunyaviridae Marburg – filoviridae (?) Ebola – filoviridae (?) |
|
|
What are anti-idiotype vaccines?
|
Idiotype - unique amino acid in Ab- a mirror of the epitope in the antigen
Anti-ids raised against antibodies to HBsAg elicit anti-viral antibodies. |
|
|
What are the important factors the influence infiltration and spread in a given population?
|
1. Population density (direct contact- aerosol, sexual ) /indirect contact- common water supply)
2. The critical population size (isolation) 3. Clinical status (babies, elderly, malnutrition) 4. Seasonal variation (Arbo/summer, adeno, rhino/spring, corona,,RSV/winter) 5. Other factors? -Lessons from accidental infections ( World war II :HBV contaminated vacc (YF) , 1955: Polio virus inactivated vacc. ,1950: Rabbit myxoma virus planned to kill rabbits in Australia) |
|
|
What is a recombinant vector? What are advantages and disadvantages of using them for vaccines?
|
A gene for viral protein is inserted into a non pathogenic organism or live attenuated virus (vaccinia) and is transferred as vaccine
Advantages- Combination of live attenuated and inactivated vaccine Antigen is present as the original virus does Humoral and cell mediated responses can be induced by the vector Can posses antigen for multiple viruses (vaccinia virus) Some strains are safe for immunodeficient persons. Disadvantages- Immunity for the vector- restricted use Not an optimal immunity- still under development |
|
|
How can known viruses expand their niche?
|
1. Disease of modern sanitation (poliovirus in the early 20th century
2. Disease of exploration and colonization (lethal epidemics of measles and smallpox). Explosive epidemic spread due to viruses finding susceptible hosts in the larger population that have never been exposed (the evolving host-virus interaction). 3. Human disease resulting from genetic reassortment of viruses in animals (influenza A virus as an example). Influenza is the paradigm for a situation in which continued evolution of the virus in several host species is essential for its maintenance. 4. Human disease resulting from changing climate and animal. Zoonoses are often classic cases of emerging viruses that reflect our changing environment (hantavirus zoonotic infection, filaviruses, caliciviruses) |
|
|
What are the problems in developing an anti-HIV vaccine?
|
Problems in developing anti HIV vaccine:
Virus can hide in cells- Latency Cell-cell transmission Lack of animal models Polymorphism/hypervariability: DRIFT killed HIV-1 does not retain antigenicity The use of a live retrovirus vaccine raises safety issues Activation of same cells that virus infects |
|
|
Explain how exploration and colonization affects viruses?
|
Disease of exploration and colonization (lethal epidemics of measles and smallpox). Explosive epidemic spread due to viruses finding susceptible hosts in the larger population that have never been exposed (the evolving host-virus interaction).
|
|
|
Explain the DNA vaccines? How are they introduced? How do they work?
|
Introduction of a DNA plasmid into the vaccinee- in vivo transfection and expression of an antigen that causes an immune response.
Expression of viral antigen in cells in vivo The plasmid does not replicate in the cells - only proteins are produced The plasmid DNA is introduced - injection to the muscle cells - into skin - bombarding with DNA-coated gold particles - into nasal tissue in nose drops |
|
|
Which flu is classified as endemic virus? Why? How?
|
Influenza A and B
Antigenic drift Mutations in HA or NA New strain emerges |
|
|
What are the advantages of DNA vaccines?
|
Easily manufactured in large amounts, stable, cheap
A DNA sequence can be changed easily in the laboratory in response to changes in the infectious agent Expression in the vaccinee -> the antigenic protein(s) produced in the same way as the viral proteins ((humoral and cellular) Mixtures of plasmids could be used -broad spectrum vaccines The plasmid does not replicate and encodes only the proteins of interest-safe |
|
|
Which flu is classified as pandemic? How does it work?
|
Influenza A
A new hemagglutinin and/or a new neuraminidase Antigenic shift New subtype emerges |
|
|
What are biofilms? Where are they located? What are they made of? What kind of behaviors do they exhibit?
|
complex aggregation of microorganisms marked by excretion of a protective and adhesive matrix
have them on teeth, tongue, drinking water, medical implants Single-celled organisms generally exhibit two distinct modes of behavior. The first is the familiar free floating, or planktonic, form in which single cells float or swim independently in some liquid medium. The second is an attached state in which cells are closely packed and firmly attached to each other and usually a solid surface. The change in behaviour is triggered by many factors, including quorum sensing, as well as other mechanisms that vary between species. When a cell switches modes, it undergoes a phenotypic shift in behavior in which large suites of genes are up- and down- regulated. |
|
|
What is the difference b/w antigenic shift and drift?
|
Antigenic shift: reassortment between human and non human virus in a nonhuman host cell.
Antigenic drift: accumulation of mutations that facilitate evasion from the host immune system. |
|
|
How are stains used in parisitology? What are some examples?
|
he first anti-parasitic chemotherapies were developed based on stains that were used to selectively bind to pathogenic agents.
Ex: Methylene blue stains plasmodium parasites at a lower concentration than it stains leukocytes, allowing it to be used in plasmodium identification. Trypan red was first developed as a stain for trypanosomes. Because these stains were already known to selectively bind to parasites, they were good research targets for drugs. |
|
|
What is H5N1? What is its origin and history? Where was it located? How to prevent an emerging new strain?
|
An outbreak of Avian Influenza H5N1 occurred in Hong Kong in 1997 where 18 persons were infected of which 6 died.
The source of the virus was probably from infected chickens and the outbreak was eventually controlled by a mass slaughter of chickens in the territory. All strains of the infecting virus were totally avian in origin and there was no evidence of reassortment. However, the strains involved were highly virulent for their avian hosts. 2002- highly pathogenic H5N1 in waterfowl, Hong Kong; 2003 - two families in Hong Kong; 2004 – poultry outbreak in China and 23 deaths out of 34 infected people in Thailand and Vietnam |
|
|
How are antiprotozoal agents used? What are their targets? What about antihelminthic?
|
Antiprotozoal agents are usually targeted to proliferating cells. Targets include:
1.Nucleic acid synthesis 2.Protein synthesis 3.Metabolic pathways Antihelminthic agents are mostly targeted at the non-proliferating adult worms. Targets include: 1.Neuromuscular molecules 2.Carbohydrate metabolism 3.Microtubular integrity |
|
|
What are the carrier states for s. aureus?
|
Nasal carraige (though also in GI, perineal, throat).
20% persistant 60% transient 20% never Also nosocomial transmission |
|
|
What is metronidazole? How is it used? What can it fight? How does it work? How is it administered?
|
Metronidazole--used against amoebas, other protozoons, and some bacteria. It is used to treat H. pylori infection. It is also used against trichomoniasis and giardiasis.
Once it is diffused into the cells, the nitrole group on the drug is reduced by electron transport proteins, which generates the active form of the drug. In other words, metronidazole is administered in its inactive form, and needs activation by anaerobic reduction. |
|
|
What are some drugs that act in the lumen of the gut, other than metronidazole? What are they made of? Are they absorbed? Where do that act?
|
Other drugs that act in the lumen of the gut and that are used against extracellular amebiasis include:
1.Paromycin--an aminoglycoside 2.Iodoquinol --an 8 hydroxyquinoline compound These drug are not absorbed by the cells, and act on the mucosal lining of the gut. |
|
|
What are the risk groups with high carriage rates of aureus?
|
Diabetes Mellitus
Dialysis Patients HIV Chronic Skin Diseases IV Drug Abusers |
|
|
What is the problem with malaria treatments? What drug is used now? What is its problem? How does it work?
|
Problems with malaria treatment include:
1.Short half life of available drugs (a problem in artemesinin derivatives) 2.Length of time the drug needs to be taken to be effective 3.Development of resistance 4.Side effects (ex: mefloquine, which is taken as a prophylactic against malaria, has been associated with neuropsychiatric problems) 5.Expense Currently, the major drug of choice for malaria is chloroquine. The major problem with chloroquine is that most plasmodium strains have developed resistance, especially those in SE Asia (less so for African strains). Chloroquine acts by interfering with the detoxification of heme. Heme is a toxic byproduct of hemoglobin digestion that plasmodia control by causing it to form aggregates inside food vacuoles, which greatly lowers its concentration inside the parasite. Chloroquinone blocks the aggregation of heme, allowing the heme to poison the parasite. |
|
|
What does aureus look like? What is its type? What is its important virulence factor?
|
Gram pos cocci that appear as grape-like clusters.
Catalase positive (important virulent factor) |
|
|
What are some antimalarials to which parasites have resistance?
|
1.Primaquine--inhibits the electron transport chain. Parasites develop resistance by increasing their mitochondria concentration.
2.Sulfonamides--inhibit PABA, which is important in many biosynthetic pathways. Resistance develops by gene amplification. 3.Proguanil and pyrimethamine--inhibit DNA synthesis by inhibiting dihydrofolate reductase. Resistance develops by a mutation in the drug's binding site. |
|
|
What are the three different species of staph? Distinguish and differentiate them.
|
i. staph aureus- of 3 most important
1. distinguished from others by coagulase production – enzyme causes plasma to clot via clotting cascade 2. ferments manitol and hemolyzes RBC (others don’t) 3. >90% contain plasmid for β-lactimase (enzyme that degrades many PCN) 4. some strains are also resistant to β-lactimase resistant PCN --> a. MRSA (methicillin resistant staph aureus) b. NRSA (nafcillin-resistant staph aureus) 5. VISA- vancomycin intermediate staph aureus a. resistant to vancomycin ii. staph epidermis 1. coagulase negative iii. staph saprophyticus 1. coagulase negative |
|
|
What is the major drug used against helminthes? Which diseases does it effectively fight? How? What are some other drugs used? Is there resistance to any of these drugs?
|
Lyermectin
cts by binding to a glutamate-gated chloride channel in the helminth's nerve and muscle cells, and may also interact with other ligand-gated chloride channels. It is effective against several parasitic disease, including: 1.Onchocerciasis ("river blindness") 2.Lymphatic filariasis (elephantitis--blockage of the lymph vessels) Avermectins are derivatives of Ivermectin that are also used against helminths. Praziquantel is another drug, widely used against trematodes and cestodes. There is no known helminth that has developed resistance against antihelminthic drugs used in humans (although resistance has developed in veterinary medications, which means that it will almost certainly develop eventually against human antihelminth medication). |
|
|
What diseases do the different strains of aureus cause?
|
1. staph aureus- causes abscesses, pyogenic infections (endocarditis, septic arthritis, osteomyelitis), food poisoning, TSS
most common causes of hospital acquired pneumonia, septicemia, and surgical wound infections 2. staph epidermis- can cause endocarditis and prosthetic joint infections 3. staph saprophyticus- causes UTIs |
|
|
What two types of bacterial growth exist? Describe them.
|
1.Planktonic--a free-floating form, in which independent cells swim around in a liquid medium
2.Benthic--an attached state, in cells grow in close proximity to each other, attached to some solid surface, and communicate with each other via various signaling molecules. When a cell switches from the planktonic to the benthic form, it senses the change through a mechanism called quorum sensing, and undergoes phenotypic changes resulting from the activation of specific genes. |
|
|
What are the cell surface determinants of aureus virulence?
|
1. Protein A- major protein in cell wall
protects organism from opsonization and phagocytosis 2. teichoic acid- mediate adherence of staph to mucosal cells . help induce septic shock 3. polysaccharide capsule- important virulent factor poorly immunogenic (makes vaccine creation difficult) 4. surface receptors- permit “phage typing” of strains for epidemiologic purposes 5. Microcapsule (small amount of polysacchrise capsule) – found of most strains prevents phagocytosis 6. peptidoglycan- has endotoxin-like properties can stimulate macrophages to produce cytokines and activate complement and coagulation cascades |
|
|
Describe plaque formation. What type of bacteria are involved?
|
1.Lots of molecules from the saliva adhere to the teeth.
2.Bacteria colonize the teeth by a combination of hydrostatic interactions and recognition of epitopes on the teeth. These original bacteria are mostly streptococci. 3.As the streptococci grow outwards, other bacteria form colonies on top of them, in a phenomenon called co-aggregation. 4.A biofilm of many different bacteria adhering to each other begins to form. Fusobacteria--bacteria that adhere to the biofilm, and allow many other bacteria to stick to them. |
|
|
What are the staph toxins?
|
1. Exoenzymes (excreted enzymes): proteases, lipases
2. Toxins: Hemolysins, PVL, Exfoliative toxins (ETA, ETB), TSST-1, SEA-SEO |
|
|
What are the stages of biofilm formation?
|
1.Adhesion of bacteria to a surface via hydrophobic or electrostatic interactions, or adhesion of bacteria to particular epitopes on host cell, mostly sugar moieties.
2.Proliferation--during this stage, the bacteria sense that they are on a surface, and undergo physiological changes 3.Enmeshment--production of extracellular polymers that create a molecular gel around the bacteria. The internal region of the extracellular matrix will be more favorable for anaerobic bacteria. 4.Communal action--the stage in which bacteria may cause disease (for example, by secretion of harmful enzymes 5.Dispersal--when the biofilm gets too crowded, some bacteria become planktonic and head off in search of new environments |
|
|
What is TSS? What different types are there? How does the toxin work>
|
1. Menstrual -- associate with use of high absorption tampons. Due to TSST-1
2. Non-menstrual -- can be due to TSST-1, SEB, SEC TSST is super-antigen that causes toxic shock that stimulates release of IL-1, IL-2 and TNF |
|
|
How does P. aeruginosa utilize biofilm?
|
P. aeruginosa can attach to a surface and upregulate a cluster of genes that produces alginase, the molecular glue, within fifteen minutes of attachment.
|
|
|
What is the mechanism for MRSA?
|
1. Horizontally transferred DNA element -- SCCmec
2. Site speicific recombination 3. mecA gene encodes PBP2a, which is capababble to cell wall syntheis. Stops PBP from binding |
|
|
What are ways in which bacteria know that they are on a surface?
|
1.Limited N supply
2.Membrane perturbation, due to i.High osmolarity ii.ethanol |
|
|
What is What is mecA? How is it transmitted?
|
It is a part of a large, mobile genetic element (SCCmec -- resembles pathogenicity island, but with no virulence factors). Transmitted sometimes by phages. Not a plasmid. Chromosomal and difficult to transmit.
|
|
|
How can biofilms be inhibited?
|
1.Modifying the surface so that bacteria cannot stick to it (has not yet been successfully done)
2.Compete with bacterial adhesion 3.Break away bound cells, either mechanically or chemically e.g. Use oil droplets to remove hydrophobic bacteria bound to the mouth 4.Use toxins or antibiotics 5.Interfere with signaling 6.Replace the infected surface, if possible |
|
|
What is the Ccr complex? What is the Mec complex?
|
Look this up.
|
|
|
What are nosocomial infections? Examples?
|
result from medical treatment, and are secondary to the patient's initial condition. The use of invasive devices increases the chance of a nosocomial infection, particularly because bacteria stick very easily to plastic.
Candida is one of the most common nosocomial (fungal) infective agents. |
|
|
What is the origin of SCCmec and the mec gene?
|
Horizontal transfer from SCN, Scuiri, enterococcus hiriae
|
|
|
What are risk factors for MRSA? CA-MRSA?
|
MRSA: previous contact with health care sytem, longer hostpitalization
CA-MRSA: we have no idea. Cloncal spread caused be lapses in IC |
|
|
What is Trimethylamine urea (TMAU)?
|
rare genetic disease, caused by a molecule that builds up in the bloodstream and is a waste product of choline that normally should be broken down by oxidases. This molecule exists at low concentrations and people can taste it (it makes them think they smell like rotten fish) but most other people cannot smell it.
|
|
|
What is US300>
|
The most common single clone of CA-MRSA
SCCmec IV Resistant to ciprofloxacin |
|
|
What is panton valentine leukocidine?
|
A pore forming cytotoxin
Strains containing pvl genes were associated w/ser=vere SST infections |
|
|
What is ACME -- arc gene cluster?
|
It is on the US00
Arginine catabolic mobile element -- virulence/strain survival factor Different from native arc gene carred by all aureus. highly similar to ACME from s. epideridis Inhibits NO production, allow survival in low pH, and anaerobic conditions (argiinine deaminase pathway) enhances fitness |
|
|
What are examples of CCR5 antagonists? How do they work?
|
1. Selzentry, Maraviroc -- A selective, small molecule CCR5 antagonists
2. Vicriviroc -- A noncompetitive allosteric antagonist of CCR5 |
|
|
What is an example of a CXCR4 antagonist? How does it work?
|
AMD-3100-
Small molecule inhibitors bind to CXCR4 and blocks the interaction between CXCR4 and the V3 loop of gp120 |
|
|
In terms of HIV, what is an example of a fusion inhibitor? What does it work? What does it interact with?
|
Enfuvirtide (Fuzeon)-T-20
36 residue synthetic peptide that inhibits HIV-1 fusion with CD4 cells Interact with gp41 Blocking fusion of gp41 with cell membrane |
|
|
What are anti-F protein antibodies? How can these be used as an antiviral? What is an example?
|
Fusion inhibitor. Stopping the binding of TLR 4 to the RSV.
Synagis, palivizumab Neutralizing and fusion-inhibitory activity Ab against Respiratory Syncytial Virus (RSV) Licensed for the prevention of RSV infection in high-risk infants and children (serious lung infections) |
|
|
What are examples of viral uncoating inhibitors? (Influenza) How do they work? What are their limitations?
|
Amantadine, Rimatadine.
Block the proton channel activity of the influenza A viral M2 protein. Inhibit the acid-mediated dissociation of the RNA complex early in replication. Limitations: lack of activity against influenza B, toxicity, resistance |
|
|
What is the life cycle of a picornavirus? What drugs can be used to inhibit? What do they work on?
|
1. Attachment to 150S
2. Conformational Transition 3. Uncoating Inhibiting of picornaviruses uncoating Pleconaril --Rearrangement of capsid proteins- Stop the conformational change. Fits into a hydrophobic pocket in the nucleocapsid and interrupts the replication of the virus |
|
|
What is thmidine thymidine kindase in viruses? Which families uses them? Are there differences in TKs by group? If so, how?
|
Viral thymidine kinase allows the virus to grow in cells that do not have a high concentration of phosphorylated nucleic acid precursors
Herpes, Pox. The thymidine kinase (TK) encoded by HSV or VZV has a rather broad substrate specificity. (aciclovir and ganciclovir). EBV TK and HHV-8 TK have a narrower substrate specificity with a clear advantage for thymidine-derived nucleoside analogues |
|
|
How does acyclovir work? What does it do? How is it taken? What is it for? Against which virus does it lack activity?
|
Inhibits viral DNA synthesis :
- Competition with dGTP-inhibition of DNA polymerase - Chain termination following incorporation into the viral DNA Viral DNA polymerase is more sensitive than cellular DNA polymerase It is taken orally, topically or intra-venously HSV-1, HSV-2 and VZV lack of activity against CMV |
|
|
How does ganciclovir work? What does it do? How is it taken? What is it for? Is it specific for a certain virus?
|
Targets - viral DNA polymerase- a chain terminator
Phosphorylation by the viral: thymidine kinase- herpes virus protein kinase- UL97-CMV (specificity ) Viral polymerase has higher affinity for Ganciclovir than the host enzyme Ganciclovir is mutagenic, its use is limited to patients with serious CMV infections |
|
|
Name the oral prodrug of the following:
1. Acyclovir 2. Penciclovir 3. Ganiciclovir |
1. Valaciclovir
2. Valganciclovir 3. Famciclovir |
|
|
How does adensosine arabinsoside work? What does it do? How is it taken? What is it for? Is it specific for a certain virus?
|
Nucleoside analog.
A complete sugar but it is arabinose rather than ribose Phosphorylation by cellular or viral kinases Inhibiting acyclovir-resistant/TK-deficient mutants of HSV and VZV Less efficient and more toxic than acyclovir. Severe side effects is only used in potentially lethal disease |
|
|
Of the thymidine analogs, what is special about Idoxuridine and Brivudin?
|
1. Idox -- lacks selectivity, only for topical treatment of HSV keratitis
2. Briv -- Phosphorylated by viral thymidine kinase, potent selectively against HSV-1 |
|
|
What is Cidofovir? How does it work? What does it inhibit? What is it active against?
|
A chain terminator
Competitive inhibitor (dCTP); DNA polymerase inhibitor Inhibits the DNA polymerases at lower concentration needed to inhibit human DNA polymerases it inhibits acyclovir-resistant thymidine kinase Active against: herpes viruses; CMV; poxviruses, BK virus( polyoma virus), HPV (human papillomavirus) adenoviruses. Resistance- viral DNA polymerase |
|
|
What is zidovudine? How dies it work? What does it treat? It is administered in combination with anything else? Why or why not?
|
NRTI. A chain terminator.
Phosphorylated by a cell kinase – Reverse transcriptase (RNA-dependent DNA polymerase) is more sensitive to the drug than human DNA-dependent DNA polymerase High mutation rate -emergence of resistant viral mutants- AZT is administered in combination with other drugs. anti-HIV, severe toxicity effects |
|
|
What is Lamivudine? What does it do? What is it active against?
|
NRTI.
An analogue of cytidine Needs to be phosphorylated Chain termination during reverse transcription Inhibits also cellular DNA polymerase Active against HIV types 1 and 2 and hepatitis B virus low affinity for human DNA polymerases -Low toxicity |
|
|
What is special about nNRTI? What are some examples? What are their mechanisms?
|
The most potent and selective reverse transcriptase inhibitors
Non-competitive inhibitors Target an allosteric pocket on the RT Work synergistically with nucleoside analogs such as AZT Work against nucleoside-analog resistant HIV Resistant mutants arise rapidly (little use in monotherapy) Binds directly to viral RT, disruption enzyme's catalytic site: Delaviridine, Nevirapine, Efavirenz |
|
|
What is Foscarnet? how does it work? What does it inhibit? Does it require phosphorulation? What does it act against? Does it work syngeristically with any other drugs?
|
non-nucleoside inhibitor
A competitive inhibitor of DNA polymerase Selective inhibition at the pyrophosphate binding site on virus-specific DNA polymerases Does not require phosphorylation for its antiviral activity Inhibits replication of herpes viruses ;CMV; HSV types 1 and 2, HIV Useful when virus gained resistance to other drugs, Acyclovir. |
|
|
What are characterisitcs of nucleoside analogs? How do they work? What do they require?
|
Must be activated through phosphorylation steps
Compete with the natural substrates of the DNA polymerase or reverse transcriptase reaction Can inhibit the incorporation of the natural substrates into the growing DNA chain Can become incorporated into DNA- chain terminators |
|
|
What are characterisitc of NRTIs? What are the steps of their activation? How do they act? What do they lead to?
|
Three phosphorylation steps, catalyzed
by cell-derived kinases, to be converted to their active metabolites Act as competitive inhibitors or alternative substrates with respect to the normal substrates (either dATP, dGTP, dCTP or dTTP) lead to the termination of chain elongation |
|
|
What is the defining characteristic of nucleotide reverse transcriptase inhibitors?
|
Presence of the phosphonate group- only two phosphorylation steps by cellular kinases ( tenofovir )
|
|
|
What are characterisitcs of HIV non-nuceloside RT inhibitors? What do they interact with?
|
Interact with an allosteric binding site on HIV‑1 reverse transcriptase that becomes exposed upon ligand binding
potent, favorable safety profile and ease of dosing Relatively rapid emergence of resistance nevirapine, delavirdine and efavirenz |
|
|
What is Ribavirin? How does it work? What does it treat?
|
RNA synth inhibitor.
A nucleoside anti-metabolite antiviral agent blocks nucleic acid synthesis -used against both RNA and DNA viruses. act as a guanosine analog, inhibitors viral mRNA syth. Treatment of chronic HCV, respiratory syncytial virus (RSV), influenza A and B |
|
|
What are the mechanisms that ribavirin triphosphate engages in? What viruses are affected at each step?
|
Inhibition of viral capping
-viral guanylyltransferase or methyltransferases Inhibition of RNA-dependent RNA polymerase activity (HCV) Inhibition of the viral helicase activity (reoviruses) Incorporated into the viral genome causing lethal mutagenesis (poliovirus) |
|
|
What are some HIV protease inhibitors? Which is most potent? How does it work?
|
Amprenavir
Nelfinavir Lopinavir Atazanavir Bevirimat ritonavir is a very potent inhibitor of cytochrome P450 3A4, the enzyme responsible for metabolism of most protease inhibitors |
|
|
What are inhibitors of influenza neuaminidase? How do they work?
|
Sialic acid analog- acts as a transition-state analogue, preventing progeny virions from emerging from infected cells
Zanamivir Oseltamivir |
|
|
What are some recombinant delivery approaches for HIV?
|
Murine retroviruses
Lentiviruses Adeno-associated virus (AAV) |
|
|
What are some anti-HIV gene therapy approaches?
|
(i) RNA-based agents
antisense, ribozymes, aptamers and RNA interference (RNAi) (ii) protein-based agents dominant-negative proteins, intrabodies, intrakines, fusion inhibitors |
|
|
How are antisense RNA transgenes used? What are advantages? Examples?
|
Antisense molecules to:
Structural proteins : HIV-1 gag and env Regulatory Tat activation region (TAR) The Rev responsive region (RRE) A short anti–U5-region High affinity for the target gene mRNA Minimal side effects and a lower probability of emergence of drug resistant viral variants Relatively lowering development and production cost VRX496- an antisense HIV envelope gene that has been transduced into CD4+ cells |
|
|
What is formivirsen?
|
Antisense RNA transgene.
A 21-base phosphorothioate oligionucleotide It is complementary to the mRNA for the major immediate-early transcriptional region of CMV Active against CMV strains resistant to ganciclovir, foscarnet, and cidofovir. The first FDA-approved |
|
|
What are ribozymes? How can they be applied for gene inhibition?
|
Antisense RNAs that enzymatically cleave targeted mRNAs
Including viral U5 region, tat and rev Rz2 - HIV-1 tat gene ribozyme |
|
|
What are advantages of using siRNAs in gene therapy? Challenges?
|
Advantage over traditional antisense method - significantly enhanced potency , lower concentrations needed
A large number of potential targets The development of RNAi therapeutics can begin once a viral genome sequence is known Challenges for RNAi-based therapies: Viruses accumulate mutations, particularly RNA genomes viruses accumulation of resistant mutant viruses have evolved efficient suppressors of RNAi ( vaccinia E3L and influenza NS1 proteins and HIV-1 Tat ) |
|
|
How can siRNAs be used against CCR5?
|
CCR5-siRNA expression high inhibition of CCR5 synthesis on the cell surface
Introduced into human T lymphocytes Protected lymphocytes from HIV-1 virus infection |
|
|
What are RNA decoys? What do they induce? What are possible side effects?
|
Provide competing binding sites for transcriptional activators or mRNA stabilizing elements
-induce mRNA instability-destruction of the mRNA -HIV TAR- binds and sequesters Tat -HIV RRE -binds and sequesters Rev Short RNA decoys (13nt) minimal binding domains of HIV-1 RRE Cellular factors associate with these structures may be sequestered - undesired side effects |
|
|
What is HIV RevM10? How is it used?
|
A dominant-negative mutant of the HIV-1 Rev trans- activator protein.
Block the export of singly spliced and unspliced HIV RNA from the nucleus to the cytoplasm --> preventing packaging and subsequent transmission One of the most potent inhibitors of HIV replication |
|
|
What is M87? How is it used? Has it been shown to be effective?
|
Fusion inhibitor, preventing viral entry.
M87- Encodes the membrane-anchored antiviral peptide C46, derived from the the HIV-1 envelope glycoprotein gp41 (contains T20 sequences) A pilot clinical trial: Infusion of CD4+ T cells transduced with: Retroviral M87 vector- viral loads were not affected The M87 inserted in a lentiviral vector was effective in preclinical studies |
|
|
What family does SARS belong to? What it its host range?
|
Corona (enveloped, +ssRNA)
Mammalian, Avian Gene: HCoV-OC43 |
|
|
What are the properties of small pox that led to its eradication?
|
a. viral characteristics:
i. exclusive human host range (no animal reservoir or vector) ii. single serotype iii. animal and human poxvirus share antigenic determinants (“Safe” live vaccines prepared from animal poxvirus) b. Disease characteristics: i. consistent disease presentation with visible pustules (ID of source of contagion allowed for quarantine and vaccination of contacts) c. Vaccine stable, inexpensive, easy to administer i. presence of scar indicating successful vaccination |
|
|
There are 8 genuses of pox that can affect humans. Name three that we need to care about.
|
1. Orthopox
2. Parapox 3. Molluscipox |
|
|
What is monkey pox? Where is it found? Cow pox? What genus do these belong to? Can they be used for vaccination? What shape are the virion?
|
a. monkeypox- causes similar disease to small pox
i. mainly found in central Africa ii. infection by contact with wild animals 1. person-to-person infection may occur iii. smallpox like disease iv. vaccination with vaccinia protects b. cowpox- localized disease infection from cows i. local hemorrhagic lesions Yes, can be used for vaccination. Brick-shaped |
|
|
What are some diseases of the parapox genus? Can this family be used for immunization? What shape are the virion?
|
parapoxvirus- viral infection doesn’t induce complete immune protection
1. milker’s nodes- localized lesion from cow infection 2. Orf- localized lesion from sheep infection Ovoid-shaped. |
|
|
What is molluseum contagiousum?
|
disease causing lesions (wart-like) all over body (STD)
a. strictly human virus b. incubation from months – years c. can be eliminated by nitrogen freezing |
|
|
What is the structure of a pox virus?
|
iii. complex structure --> core, lateral bodies, outer membrane and sometimes envelope
1. DNA and viral enzymes within core membrane 2. 2 lateral bodies found next to core membrane 3. outer envelope- really an inner envelope made of viral components from viral replication 4. real outer envelope- membranes which originate from golgi membrane (that is acquires from host) iv. virus comes equipped with some enzymes needed for initial replication 1. this is b/c they are DNA viruses that replicate in cytoplasm |
|
|
How can small pox be diagnosed?
|
a. throat swab, freshly open pustule ,CSF
i. EM --> all orthopox virus virions have same appearance than can be distinguished from parapocvirion ii. inoculation of the chorioallantoic membrane (CAM) – 1. cowpox virus --> produces hemorrhagic lesions 2. small pox --> small opaque white lesions 3. vaccinia --> large opaque white lesions 4. Molluscum contagiosum and tanapoxvirus --> do not grow on CAM iii. cell culture- virus grows in cell cultures b. TODAY --> ELISA and PCR |
|
|
What are the common presentation of s. pneumoniae?
|
Pneumonia
Meningitis Sinusitis Otitis media Fever of unknown source in children. |
|
|
What is the structure of s. pneumoniae? How does it grow? How does it replicate?
|
Gram positive diplococci
Replicates in chains Encapsulated Catalase negative, but produces H2O2 Produces α-hemolysis. Growth is inhibited by ethyl hydrocurprine (optochin). Is lysed by bile salts. |
|
|
Is s.pneumoniae part of the normal flora? Where does it stay? What are risk factors? How is it transmitted?
|
Carried by 90% of children at some stage until age of 4y
Carriage is the first step towards infection and the source of spread in the community Most common place of colonization – nasopharynx, in adults also pharynx |
|
|
What are the virulence factors of s. pneumoniae?
|
Capsule
Pneumolysin H2O2 Pilus |
|
|
How is peroxide used as a virulence factor? What does it do? Does it work synergistically with anything?
|
H2O2->increase mutation rates in S. pneumoniae
Cytotoxic effects on human epithelial cells in culture and host tissue in animal models. Synergistic with pneumolysin -> induces apoptosis of cells. Bacterial competition: Inhibit/kill other inhabitants (even catalase –expressing bacteria). Endogenous between 0.1mM-1mM does not inhibit S. pneumoniae growth. |
|
|
What is pneumolysin?
|
Pore-forming toxin
Member of cholesterol binding toxins Released from bacteria as monomeric protein. |
|
|
What is the mechanism of pore formation for pneumolysin?
|
After release from bacteria:
Assemble into pre-pore oligomers on the surface of cholesterol containing cell membranes. The prepore puncture the membrane and form large pores. |
|
|
What is special about the pnemoniae pilus?
|
Adherent and inflammatory determinant
Only 25% of Sp strains carry a pilus Most piliated strains are vaccine-type strains |
|
|
What are two anti-pneumonococcal vaccines?
|
Capsular polysacharide vaccine
Conjugated pneumococcal vaccine (PCV7, PCV11, PCV13). |
|
|
What is an example of capsular polysaccharide vaccine? What is it? How does it work? What are its problems?
|
Pneumovax®23
Highly purified capsular polysaccharides from 23 most prevalent types. Accounts for > 90% of blood isolates. Problems: Does not induce T cell response Not effective in children <2 y Effectiveness in elderly questionable (only in bacteremia, not pneumonia) |
|
|
What is an example of a pneumococcal conjugated vaccine? How many serotypes are involved? Is it efficient?
|
Polysacharides of 7 common serotypes conjugated to a protein carrier
Includes 7 serotypes 4, 6B, 9V, 14, 18C, 19F, 23F High cost, not available in low income countries Highly efficient in US: 77% decrease in invasive pneumococcal disease (in children<5y) 39% decrease in Hospital admissions (children <2y) |
|
|
How is MRSA spread?
|
Spread is mainly clonal. Only few clones are the cause of most infections.
Major cause for clonal spread: lapses in IC Yet - role of Ab pressure:… |
|
|
How did CA-MRSA evolve?
|
“Hospital escape” of unsuccessful HA-MRSA
|
|
|
What is the arc gene clouster? What pathway does it use? What does it do?
|
Arginine Catabolic Mobile Element: virulence/strain survival factor
Different from native arc gene carried by all S. aureus Highly similar to ACME from S. epidermidis Arginine deiminase pathway Inhibits the nitric oxide production Allows survival in low ph, anaerobic conditions Enhances fitness: enhances potential to grow and survive within a host |
|
|
How can CA-MRSA be treated? HA-MRSA?
|
CA-MRSA
Clindamycin ?? (high ery-R suggests inducible clinda-R) TMP-SMX? Rifampin? Vancomycin HA-MRSA Vancomycin Linezolid Daptomycin |
|
|
What is GAS?
|
Gram positive cocci in pairs or short chains. In media may form long chains.
Produce capsules of hyaluronic acid. Several virulence factors (Somatic and extracellular excreted). |
|
|
What are GAS' virulence factors? What is the M protein?
|
Hyaluronic acid capsule (avoid opsonization).
Complex cell wall containing many antigenic substances. M protein – major somatic virulence factor. Rich of the protein = resistant to phagocytosis No M protein exression = avirulent emm gene encodes the molecule. >90 M types are recognized. |
|
|
What are strep's excreted virulence products? Describe. What are examples?
|
Streptococcal pyrogenic exotoxin (erythrogenic toxin) -> rash (Spe A-F) – Superantigens.
Hemolysins (lyse erythrocytes, leukocytes and platlets): Streptolysin O (oxygen labile) (ASO ab – indicate recent streptococcal infection) Streptolysin S (produced when grown in Serum) (non antigenic) Damages membranes similar to ASO. β hemolysis. Extracellular enzymes: DNases (degrade free DNA->reduces viscosity of abscess material) Hyaluronidase Streptokinase (lyse clots) |
|
|
What are different invasive GAS syndromes?
|
Septicemia
Necrotizing Fasciitis Myositis Strep toxic shock syndrome (TSS) |
|
|
What is the definition of TSS?
|
Isolation of GAS from sterile/nonsterile site
Clinical signs of severity: hypotension + 2 or more of: Renal failure(Cr>2) Coagulopaty Liver involvement ARDS generalized rash that may desquamate Soft tissue necrosis (NF, myositis, gangrene) |
|
|
What determines if it will be a “commensal” or flesh eating bacteria?
|
Host immune factors?
Negative association between level of anti-M1 Ab and invasive disease. Negative association between neutralizing ab to toxins and severe invasive disease. Bacterial virulence? M1 and M3 strains Yet, it was reported that the same serotype and strain that caused mild infection in 1 person caused severe STSS in another. |
|
|
How to treat strep?
|
Always susceptible to penicillin!!!
Clindamycin should be added on initial days. IVIG should be added on initial days. |
|
|
What are manifestations of Cytomegalic Inclusion Disease?
|
CNS abnormalities - microcephaly, mental retardation, spasticity, epilepsy, periventricular calcification.
Eye - choroidoretinitis and optic atrophy Ear - sensorineural deafness Liver - hepatosplenomegaly and jaundice which is due to hepatitis. Lung - pneumonitis Heart - myocarditis Thrombocytopenic purpura, Haemolytic anaemia Late sequelae in individuals asymptomatic at birth - hearing defects and reduced intelligence. |
|
|
How can CMV be diagnosed?
|
1. Histologically (owl's eye, but low sensitivity)
2. pp65 CMV antigenaemia test 3. PCR (not the best way) 4. Virus isolation 5. Serology -- IgG, IgM |
|
|
Treatment of CMV in immunocompromised patients? What about in congen. infections?
|
1. Ganciclovir, forscarnet, cidofovir
2. usually not possible to detect congen infection unless mother has symptoms. no treatment other than offered abortion |
|
|
What are the properties of HHV 6 and 7?
|
1. beta subfamily of herpes
2. Main target T lympho 3. Spread saliva and breast feeding. Remains latent in body, likely to reactivate in immunosuppressed patients. |
|
|
What are the clincal manifestations of HHV 6?
|
Roseala Infantum (b/w 4 months and 2 years) = fever, mild rash, maybe encephalitis
Detected through serology. No antiviral treatment. |
|
|
What are the properties of EBV?
|
1. Gamma herpes
2. linear DS DNA 3. Does not normally integrate into cellular DNA, but forms circular episomes 4. Infections in children 1-6, 14-20 5. Saliva |
|
|
What are the disease associations with EBV?
|
1. Infectious Mononucleosis
2. Burkitt's lymphoma 3. Nasopharyngeal carcinoma 4. Hodgkin’s disease 5. Lymphoproliferative disease and lymphoma in the immunosuppressed. 6. X-linked lymphoproliferative syndrome 7. Chronic infectious mononucleosis 8. Oral leukoplakia in AIDS patients 9. Chronic interstitial pneumonitis in AIDS patients. |
|
|
What are the disease mechanisms of EBV?
|
1. Initiates in saliva, spreads to B cells in lymphatic tissue
2. Promotes B cell growth 3. T cells kill and limit B cell grwoth, promote latency in B cells. 4. T cell response contributes to symptoms of mononucleosis |
|
|
What are the two pathways for EBV infection?
|
1. EBNA induction. Viral proteins EBNAs, LMP1, LMP2, LP will lead to nonprodcuer cells.
2. ZEBRA peptiide comes, then EA, VCA, EBNA, MA will lead to virus production |
|
|
Describe pathogenesis of EBV.
|
1. Epthlial cells of oropharynx leads to pharyngitis
2. B cell prolif leads to heterophile antibodies (recognizes antigens present on RBCs and can agglutinate RBCs in sheep -- can recognize) 3. T cell activation will lead to atypical lymphocutes (Downey cells) Disease is caused b/c of T cell activation. |

