![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
147 Cards in this Set
- Front
- Back
|
Hooke |
- he reported to the world that life's smallest structural units were "little boxes" or cells
( cell theory) |
|
|
Jenner (1760) |
1st to discover/ use vaccine (cow pox / small pox) |
|
|
Koch (1876) |
- came up with the 4 postulates
- discovered anthrax
-1st proof that bacteria caused disease
- discovered rod-shaped bacteria |
|
|
Lister (1867) |
- began treating wounds with phenol solution (carbolic acid) and kills bacteria
- this finding proved that microorganisms are the cause of surgical infections |
|
|
Pasteur (1861) |
- he reasoned that microbes in the air were agents responsible for contaminating non-living matter
- discovered pastuerization |
|
|
Van Leeuwenhoek (1763) |
- 1st discovered the invisible world of microbes& was the first to see the microbes using the rudimentary microscope |
|
|
What are the branches of 3- Domain system? |
* The branches include: Bacteria, Archaea, and Eukarya |
|
|
What classification is the 3- Domain system based on? |
- The system is based on type of cell |
|
|
Know which types of microbes belong to which branch
- E.coli belongs to what branch?
- methanogens belongs to what branch?
- Fungi belongs to what branch? |
- E. coli belongs to the Bacteria branch
- Methanogens belongs to the Archaea
- Fungi belongs to the Eukarya branch |
|
|
What is the difference with:
spontaneous generation vs Biogenesis? |
- Spontaneous generation is the hypothesis that living organisms arise from non-living matter (false)
- Biogenesis is a true hypothesis that organisms arise from pre-existing life (true) |
|
|
How does a vaccine work? |
Protects from disease or by recovery from disease is immunity |
|
|
Define biotechnology |
The use of microbes to produce foods and chemicals |
|
|
Is regular use of antibacterial products recommended for healthy individuals?
Why or why not? |
No, because antibacterial soaps have the potential to create antibiotic-resistant bacteria |
|
|
what is normal microbiota? |
- The microbes normally present in and on human body , - it prevents growth of pathogens
-produces useful substances like vitamins |
|
|
Understand the three general types of chemical bonds |
1. ionic bond
2. covalent bond
3. hydrogen bond |
|
|
What is Ionic bond? |
- a chemical attraction between ions of opposite charge
|
|
|
To form an ionic bond ?
give an example |
one ion is an electron donor
the other ion is an electron acceptor
* ex: NA + Cl - (sodium chloride) |
|
|
Define a covalent bond
give an example |
- formed by two atoms sharing one or more pairs of electrons
ex) H2 two hydrogen atoms share a pair of electrons |
|
|
Define hydrogen bonds
give an example |
- form weak links between different molecules or between parts of the same large molecule
ex) N or O |
|
|
Covalent bonds are stronger than what bond? |
Stronger than ionic bonds |
|
|
Dehydration synthesis vs Hydrolysis? |
- Dehydration synthesis = joins the monomer units , takes water out, gets rid of water molecule to make a bond
- Hydrolysis = breaks polymers down , water is split and broken |
|
|
What is the formula for carbohydrate? |
CH2O |
|
|
Sucrose formula |
C-12 H-22 O-11 |
|
|
Glucose formula |
C-6, H-12, O-6 |
|
|
Fructose formula |
C-6 , H-12, O-6 |
|
|
What are the two monosaccharides that combine to form sucrose? |
Glucose and Fructose (C-6 , H-12 , O-6) |
|
|
What are tetroses? |
- 4 carbon sugars |
|
|
What is Pentoses? |
- 5 carbon sugars |
|
|
What are monosaccharides ?
- give an example |
- are simple sugars with 3 to 7 carbon atoms
- example 1 : glucose
- example 2: fructose |
|
|
What are disaccharides? |
- two simple sugars covalently bonded
ex.1 sucrose ex. 2 lactose |
|
|
What are oligosaccharides? |
consist of 2 to 20 monosaccharides |
|
|
What are polysaccharides? |
consist of tens or hundreds of monosaccharides joined through dehydration synthesis |
|
|
Name polymers of glucose that are covalently bonded differently |
Starch, glycogen, dextran, and cellulose |
|
|
What is chitin? |
polymer of 2 sugars repeating many times |
|
|
Monomer units are joined by |
Dehydration synthesis reactions (also called condensation reactions) |
|
|
Polymers are broken down by ? |
Hydrolysis reactions |
|
|
What are carbohydrates? |
- are compounds consisting of atoms of carbon, hydrogen , and oxygen
( hydrogen and oxygen 2:1 ratio) |
|
|
Carbohydrates can be classified as ? |
- monosaccharides
- disaccharides
- polysaccharides |
|
|
What are isomers?
- give an example of two sugars |
- are two molecules with the same chemical formula but different structures and properties
ex) Glucose and Fructose
(C6 H12 O6) |
|
|
Monosaccharides may form disaccharides and polysaccharides by? |
- dehydration synthesis |
|
|
What are the 6 common elements in living organisms? |
1. Hydrogen 2. Carbon 3. Nitrogen 4. Oxygen 5. Calcium 6. Phosphrous |
|
|
What are macromolecules? |
Polymers consisting of many repeated molecules |
|
|
Name all the macromolecules |
- Carbohydrates , Lipids, Proteins and Nucleic acids |
|
|
Alcohol structure
(functional groups) |
R-OH |
|
|
Ester structure
(functional group) |
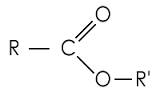
|
|
|
Amino group structure |
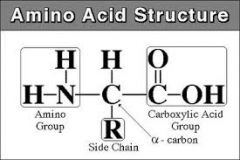
|
|
|
Carboxyl group structure |

|
|
|
Phosphate group structure |
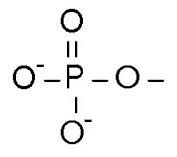
|
|
|
What are the levels of Protein Structure? |
1. The primary structure is a polypeptide chain ( Amino acid sequence)
2. The secondary structure occurs when the amino acid chain folds and coils in a regular helix or pleats (Alpha helices and B-pleated sheets)
3. The tertiary structure occurs when the helix folds irregularly, forming disulfide bonds, hydrogen bonds, and ionic bonds between amino acids in the chain.
4. The quaternary structure consists of 2 or more polypeptides. |
|
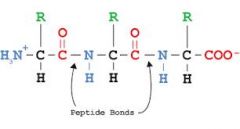
- which protein structure is this?
- What bond? |
- Primary structure
- peptide bond |
|
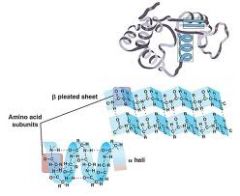
What protein structure?
(bottom pictures) |
- Secondary Structure
(bottom pictures)
( fibrous structures the alpha helix, and the beta pleated sheet) |
|
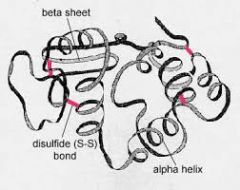
what protein structure? |
- Tertiary Structure ( Disulfide bridge , ionic bonds, H-bond, hydrophobic interactions) |
|
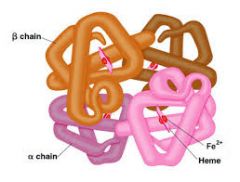
What protein structure? |
- Quarternary Structure
(2 or more polypeptides) |
|
|
Peptide bonds between amino acids are formed by? |
- dehydration synthesis |
|
|
What is the structural difference between DNA and RNA? |
DNA: Deoxyribonucleic acid
- Has H factor and thymine - Has deoxyribose - Exists as a double helix - A hydrogen bonds with T - C hydrogen bonds with G
RNA: Ribonucleic - is less stable , has OH factor b/c hydroxyl group can attack atom - Has uracil - Has ribose
- Is single-stranded - C hydrogen bonds with G |
|
|
What are Simple Lipids? |
- Are fats that consist of molecule of glycerol and 3 molecules of fatty acids
Contain only C, H, & O Also called: Fats or Triglycerides Monomer unit: 1 triglyceride + 1-3 fatty acids Formed by dehydration synthesis |
|
|
What is a saturated lipid?
- has higher melting point than? |
- has no double bonds between carbon atoms in fatty acid
- has higher melting point than unsaturated lipids
- note: the hydrocarbon tail is “saturated” with hydrogen atoms |
|
|
What is unsaturated lipid?
- what is Cis and trans? |
- has one or more double bonds
- cis: H atoms on the same side of the double bond
- trans: H atoms on opposite sides of the double bond |
|
|
Name two simple lipids? |
- saturated and unsaturated lipids |
|
|
What are complex lipids?
- give an example |
- contain such elements as phosphorus , nitrogen, and sulfur , in addition to C, H, and O
ex) phospholipids (contain one phosphate group) |
|
|
What are phospholipids? |
- are complex lipids consisting of glycerol , two fatty acids and phosphate group |
|
|
What are steroids? |
- has a carbon ring structure
- sterols have a functional hydroxyl group |
|
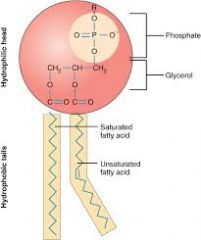
Identify this picture |
- phospholipid structure showing saturated (no double bonds) and unsaturated fatty acids (1 or more double bonds)and the molecules' polarity
|
|
|
What is the mordant used for ? |
is used in simple stains, to increase the affinity of a stain for biological specimen ( intensifies stain)
- also to coat a structure ( such as flagellum), to make it thicker and easier to see after it is stained with dye |
|
|
What is resolution? |
- the ability of the lenses to distinguish two
points at a specified distance apart (fine detail and structure)
*also called resolving power |
|
|
What is refraction? |
- is the measure of a light-bending ability of a medium |
|
|
What is the difference between basic dyes and acidic dyes? |
- Basic dye: the chromophore is cation (+)
- Acidic dye: the chromophore is anion (-) |
|
|
why are basic dyes most common? |
- Because bacteria are slightly negative charged at PH 7
-thus the colored positive ion of Basic dye is attracted to the negatively charged bacterial cell |
|
|
Give examples of Basic dyes? |
crystal violet
methylene blue
malachite green
safranin |
|
|
Why are Acidic dyes not commonly used?
- what stain is it used in? |
- they are not attracted to most types of bacteria b/c the dye's negative ions are repelled by negatively charged bacterial surface, it stains the background instead
- negative staining (valuable for observing shape) |
|
|
Compound-light microscope |
- the image from the objective lens is magnified again by the ocular lens
Total magnification = objective lens × ocular lens |
|
|
phase-contrast microscope |
- Accentuates diffraction of the light that passes through a specimen
- brings direct and reflected or diffracted light rays together (in phase) to form an image of the specimen on the ocular lens
- allows detailed observation of living organisms |
|
|
darkfield microscope |
- shows a light silhouette of an organism against a dark background
- it is useful for detecting the presence of extremely small organisms |
|
|
Flourescence Microscopy |
- specimens are 1st stained with flurorochromes and the viewed through a compound microscope by using an ultraviolet light source
- the microorganisms appear as bright objects against a dark background
- used in primarily in a diagnostic procedure called fluorescent-antibody (FA) technique or immunofluorescence |
|
|
Electron Microcscopy |
- Uses electrons instead of light
-The shorter wavelength of electrons gives greater resolution |
|
|
Transmission Electron Microscopy (TEM) |
Ultrathin sections of specimens Light passes through specimen, then an electromagnetic lens, to a screen or film Specimens may be stained with heavy metal salts 10,000–100,000×; resolution 10 pm |
|
|
Scanning Electron Microscopy (SEM) |
* 1,000–10,000×; resolution 10 nm |
|
|
Scanned-Probe Microscopy |
- uses a metal probe to scan a specimen
- Resolution 1/100 of an atom |
|
|
Atomic force microscopy (AFM) |
- uses a metal- and-diamond probe inserted into the specimen
- Produces three-dimensional images. |
|
|
Confocal Microscopy |
Cells stained with fluorochrome dyes Short wavelength (blue) light used to excite the dyes The light illuminates each plane in a specimen to produce a three-dimensional image Up to 100 μm deep |
|
|
Brightfield Illumination |
Dark objects are visible against a bright background Light reflected off the specimen does not enter the objective lens |
|
|
Two-Photon Microscopy |
Cells stained with fluorochrome dyes Two photons of long- wavelength (red) light used to excite the dyes Used to study cells attached to a surface
Up to1 mm deep |
|
|
Scanning Acoustic Microscopy (SAM) |
Measures sound waves that are reflected back from an object Used to study cells attached to a surface Resolution 1 μm |
|
|
Which microscope has the highest magnification? |
- The Transmission Electron microscope
- 10,000- 100,000 x |
|
|
Which microscope is best for 3-D microscope? |
DIC (differential interferance contrast) |
|

identify this part of the microscope |
objective lens
(primary lens that magnify the specimen) |
|
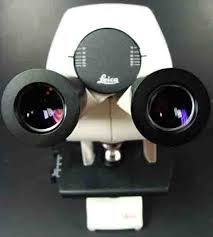
identify this part of the microscope |
- Ocular lens
* eyepiece , remagnifies the image formed by the objective lens |
|
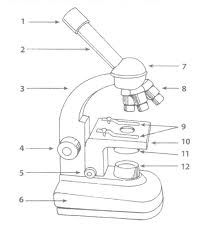
which number is the illuminator? |
- number 12 , illuminator is a light source that passes through condenser |
|

what part of the microscope is this? |
- the condenser
(focuses light through specimen) |
|
|
Review steps of gram staining |
1. Primary stain: G+ (purple) G- (purple)
2. Mordant (iodine): G+ (purple) G- (purple)
3. Decolorizing agent: G+ (purple) G-(colorless)
4. Counterstain (Safranin): G+ (purple) , G- ( Red , pink) |
|
|
Be able to calculate total magnification |
objective lens x ocular lens |
|
|
How are endospores stained? |
primary stain: malachite green
Decolorize: water
Counterstain: Safranin |
|
|
How are capsules stained? |
Mix the bacteria in a solution containing a fine a colloidal suspension of particles ( usually nigrosin) to provides a contrasting background |
|
|
What is immersion oil used for and why does it work? |
- immersion oil is used reduce light loss between slide and the lens |
|
|
What is the refractive-index? |
- is a measure of light-bending ability of a medium |
|
|
Describe Glycocalyx's structure |
- sticky layer, viscous gelatinous polymer that is external to cell wall
- can contain polysaccharide or polypeptide or both |
|
|
Function of Glycocalyx |
- It strengthens the cell surface
- helps attached cells together
- may contribute to cell-cell recognition (sugar coats) substances that surround cells |
|
|
What is flagella? |
- long filamentous appendages consisting of filament, hook and basal body
- used for motility
- consist of an arrangement of 9 pairs and 2 single microtubules |
|
|
What is Pili? |
- longer than fimbriae, only 1 or 2 per cell
Functions:
- used to bring bacteria together allowing to transfer of DNA from cell to another in a process called "conjugation"
-Twitching and gliding motility |
|
|
Fimbriae's main function |
- Help adhere to surfaces |
|
|
Gram (+)
- Gram rxn color?
- Peptidoglycan layer
- teichoic acids (y/n) ?
- periplasmic space? (y/n)
- outer membrane (y/n)
- endotoxin (LPS) (y/n) ?
|
G+
- Purple
- thick layer, 2 ring basal body
- yes has techoic acid
- no periplasmic space
- no outermembrane
- no endotoxin
- Penicillin sensitvity
- Lysozyme susceptibility
|
|
|
Gram (-)
- Gram rxn color?
- Peptidoglycan layer
- teichoic acids (y/n) ?
- periplasmic space? (y/n)
- outer membrane (y/n)
- endotoxin (LPS) (y/n) ? |
- Pink
- Thin , 4 ring basal body
- no teichoic acids
- yes has periplasmic space
- yes outer membrane
- yes has endotoxin (LPS)
- tetracycline sensistivity |
|
|
What are inclusions? |
Material held inside a cell often consisting of reserve deposits found in Eukaryotic and Prokaryotic cells |
|
|
what is the nucleus? |
- usually a spherical eukaryotic organelle frequently largest structure in cell and contains almost all the cell's hereditary info (DNA) |
|
|
What is ER? |
- Extensive network of flattened membranous sacs or tubules |
|
|
What are Ribosomes? |
- site of protein synthesis in a cell composed of RNA and protein |
|
|
What is vacuole? |
- found in plant cells
- Membrane enclosed cavities derived from Golgi complex or endocytosis |
|
|
Lysosome |
an organelle containing digestive enzymes |
|
|
Golgi |
- An organelle involved in secretion of certain proteins |
|
|
What is mitochondria? |
- primary sites of ATP production
- contain 70 S ribosomes , DNA
- multiply by binary fission
|
|
|
Plasma membrane is known to be |
selectively permeable |
|
|
Name all passive transports |
1. simple diffusion 2. facilitated diffusion 3. osmosis |
|
|
What happens in simple diffusion? |
molecules and ions move until equilibrium is reached |
|
|
What happens in facilitated diffusion? |
substances are transported by transporter proteins across membrane from areas of high to low concentration |
|
|
What happens in osmosis? |
movement of water from areas of high to low concentration across a selectively permeable membrane until equilibrium is reached |
|
|
What happens in Active transport? |
- materials move from areas of low to high concentration by transporter proteins and the cell must expand energy
* uses energy in form of ATP to more substances across the plasma membrane |
|
|
What is osmotic pressure? |
is the presence required to prevent movement of pure water ( water with no solutes) into a solution containing some solutes
*is the pressure needed to stop the flow of water across selectively permeable membrane |
|
|
Define Aquaporins |
- integral membrane proteins from a larger family of major intrinsic proteins (MIP) that form pores in the membrane of biological cells
- found in osmosis , functions as water channels |
|
|
Water molecules may pass through plasma membranes by moving through? |
- by moving through lipid bilayer by simple diffusion
- or through aquaporins (integral proteins) |
|
|
In what ways are mitochondria and prokaryotes similar? |
- Circular DNA in mitochondria and Prokaryotic organism
- Mitochondria resembles those of prokaryotes and their mechanism of protein synthesis is more similar to bacteria vs the eukaryotes
- both locations where cell energy is made
- The same antibiotics that inhibit protein synthesis on ribosomes in bacteria , also inhibit protein synthesis on ribosomes in mitochondria |
|
|
Name some characteristics of Mitochondria |
- spherical or rod-shaped organelles
- appear throughout cytoplasm of most eukaryotic cells
- powerhouse of cell , central role in ATP
- contains 70S ribosome and DNA
- has two membranes , multiply by binary fission |
|
|
What is the endosymbiotic theory? |
- 2.5 billion years ago the 1st eukaryotic cells evolved from prokaryotic cells |
|
|
What is the definition of endospore? |
- are resting structures formed by some bacteria (Clostridium and Bacillus) |
|
|
Characteristics of Endospores |
- used when essential nutrients are depleted
- highly durable dehydrated cells with thick walls and additional layers (protection)
- they formed internal to the bacterial cell membrane
- when released into environment , they can survive extreme heat , lack of water and exposure to many toxic chemicals and radiation |
|
|
What is the process that creates endospore? |
Sporulation or sporogenesis |
|
|
What are the 6 steps of sporulation? |
1. spore septum begins to isolate newly replicated DNA and small portion of cytoplasm
2. Plasma membrane starts to surround DNA , cytoplasm and membrane isolated in step 1
3. Spore septum surround isolated portion , forming forespore
4. Peptidoglycan layer forms between membrane
5. Spore coat forms
6. Endospore is freed from cell |
|
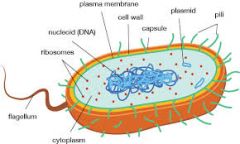
what kind of cell is this? * note be able to identify this pic |
Prokaryotic cell |
|
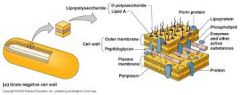
identify this cell wall |
Gram (-) cell wall
- contains lipopolysaccharides , lipoproteins and phospholipids |
|
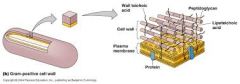
What kind of cell wall is this? |
- Gram (+) cell wall
- linked by polypeptides |
|
|
Differentiate between oxidation and reduction. Remember OIL RIG |
- Oxidation: Removal of electrons
- Reduction: Gain of electrons
*OIL RIG = oxidase is loss , reduced is Gain |
|
|
Define catabolism and anabolism |
Catabolism: provides energy and building blocks for anabolism
Anabolism: uses energy from catabolism and building blocks to build large molecules |
|
|
What factors influence enzyme activity? |
Temperature, PH , Substrate concentration, Inhibitors |
|
|
How does temperature influence enzyme activity? |
- at high temp enzymes undergo denaturation and lose their catalytic properties
- at low temp , the reaction rate decreases |
|
|
How does PH influence enzymatic activity? |
- Enzymatic activity is maximal is known as optimum PH (4-6)
- extreme acid or basic conditions will denature enzymes |
|
|
How does substrate concentration influence enzymatic activity? |
- reactions will occur more quickly as the substrate concentration increases
- the rate of reaction increases until the active sites on all enzyme molecules are filled at which point of maximum rate of rxn is reached |
|
|
How do the inhibitors influence enzymatic activity? |
1. Competitive Inhibitor
- compete with the normal substrate for active site of the enzyme
2. Non-competitive Inhibitor
- act on other parts of apoenzyme or on cofactor
- decrease the enzyme's ability to combine with normal substrate |
|
|
What are the 3 principle stages of carbohydrate metabolism? |
Glycolysis, Krebs cycle and Electron transfer chain |
|
|
What happens in Glycolysis? |
- oxidation of glucose to pyruvic acid
w/ production of some ATP and energy-containing NADH |
|
|
What happens in Krebs cycle? |
- oxidation of acetyl CoA ( derivative of pyruvic acid) to carbon dioxide
with production of some ATP energy-containing NADH and another reduced electron carrier FADH2 (reduced form of flavin adenine dinucleotide) |
|
|
What happens in electron transport chain ? |
- NADH and FADH2 are oxidized contributing, the electrons they have carried from the substrates to a "cascade"of oxidation-reduction , involving a series of additional electron carriers
* energy from this reaction is used to generate ATP |
|
|
Where is the electron transport chain located in prokaryotes and eukaryotes? |
Prokaryotes: found in plasma membrane
Eukaryotes: found contained in the inner membrane of mitochondria |
|
|
Define feedback inhibition |
Occurs when the end-product of a metabolic pathway inhibits an enzyme's activity near the start of the pathway |
|
|
For generation of ATP , define the three mechanisms of phosphorylation |
1. Substrate-level phosphorylation
2. Oxidative Phosphorylation
3. Electron transport chain |
|
|
What happens in substrate level? |
a high energy phosphate from an intermediate in catabolism is added to ADP |
|
|
What happens in oxidative phosphorylation? |
energy is released as electrons are passed to a series of electron acceptors (electron transport chain) and finally to oxygen or another inorganic compound |
|
|
What is photophosporylation? |
- the electron transfer releases energy used for synthesis of ATP
- energy from light is trapped by cholorphyll and are electrons are passed |
|
|
What is fermentation? |
- The enzymatic degradation of carbohydrates in which the final electron acceptor is an organic molecule
- ATP is synthesized by substrate-level phosphorylation and oxygen is not required |

