![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
|
What is the structure of ATP? |
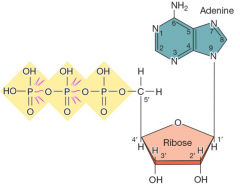
|
|
|
What is the equation for Gibbs Free Energy? |
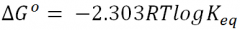
|
|
|
How much energy is released during the hydrolyzation of ATP to ADP? |
-7.3 kcal/mol. |
|
|
Here is an example of a redox reaction. |
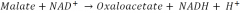
1). Malate is oxidized to Oxaloacetate. 2). NAD+ is reduced to NADH. 3). The reaction is catalyzed by Malate Dehydrogenase. |
|
|
What is the enzyme responsible for the addition of ATP to glucose, the first step of glycolysis? |
Hexokinase |
|
|
What are the two kinds of electron carriers? |
1). Freely diffusible NAD+: Nicotinaminde adenine dinucleotide, NADP+: Nicotinaminde adenine dinucleotide phosphate
2). Membrane bound (Flavoproteins, cytochromes (proteins that use an iron heme group to transfer electrons , quinones (Coenzyme Q)). |
|
|
Heterotroph |
Use reduced preformed organic compounds as carbon sources.
Animals and many microbes are heterotrophs.
Heterotrophs convert large amounts of C to CO2. |
|
|
Autotroph |
Utilize CO2 are a carbon source.
Plants and many microbes are autotrophs.
Autotrophs produce many organic compounds used by hetertrophs. |
|
|
Phototroph |
Utilize light as the source of energy. |
|
|
Chemotroph |
Oxidize chemical compounds as the source of energy.
Often from the same source of carbon. |
|
|
Litotroph |
Utilize inorganic molecules as electron donors.
In respiration, inorganic molecules will be the final electron acceptor.
Lithotrophy is unique to a few bacteria and archaea. |
|
|
Organotroph |
Utilize organic molecules as electron donors.
In fermentation, an organic compound is the final electron acceptor. |
|
|
What are the two processes by which ATP is produced? |
1). Substrate level phsphoylation 2). Oxidative phosphoylation |
|
|
What are the two functions of organic energy sources? |
1). To oxidate those energy sources for the release of energy. 2). To generate building blocks (metabolites) for anabolism. |
|
|
Amphibolic pathway |
A pathway that can function catabolically or amphibolically. |
|
|
Name a species that can perform ETC under aerobic and anaerobic conditions. |
Paracoccus denitrificans. This species also uses NO3 as the final electron acceptor. |
|
|
What is the chemiosmotic hypothesis and who came up with it? |
Peter Mitchell believed that energy released during electron transport was used to establish a proton gradient and establish a charge difference across a membrane (Proton motive force (PMF)). |
|
|
ATP Synthase (F1Fo ATPase) |
An enzyme that uses a PMF to catalyze ATP synthesis.
This enzyme is highly conserved across domains. |
|
|
What is special about the Shewanella genus? |
Shewanella can transport electrons from the ETC to things outside the cell.
Must live in anoxic environments in order to transport electrons to an anode.
May live in thick biofilms on an anode.
|
|
|
Endogenous electron acceptor |
Electron acceptors produced by the organism itself.
Ex). Pyruvate |
|
|
Exogenous electron acceptor |
Electron acceptors obtained from the environment. |
|
|
Aerobic respiration |
Utilization of O2 as the final electron acceptor. |
|
|
Anaerobic respiration |
Utilization of molecules other than water as the final electron acceptor.
Ex). NO3, SO4, CO2, and fumarate. |
|
|
Chemolithotrophy |
When an inorganic electron donor is used. |
|
|
What is a genous that uses Fe3+ as the final electron acceptor through the use of nanowires. |
Geobacter |
|
|
Dissimilatory nitrate reduction (Denitrification) |
Where NO3 is used as the terminal electron acceptor and is reduced to N2.
Species that utilizes this process are the major cause for loss of nitrogen in soil. |
|
|
Fementation |
Complete catabolism without the electron transport system and a terminal electron acceptor.
1). Occurs in the cytoplasm
2). NADH transfers hydrogen to pyruvate.
3). Generates fermentation products (lactic acid, ethanol, etc.) as well as NAD+.
4). The production of NAD+ allows for glycolysis to continue.
5). Generates ATP through substrate level phosphorylation. |
|
|
Archaea Sulfolobus |
Executes chemolithotrophy by using H2S as a source of electrons to produce sulfuric acid. |
|
|
What does the bacteria Acidithiobacillus ferrooxidans do? |
Oxidizes soluble Fe2+ to Fe3+.
O2 is used as an electron acceptor.
Forms insoluble ferric hydroxide.
Is not a very large reduction potential between Fe2+ and Fe3+ so large amounts of Fe2+ needs to be used in order to gather suitable amounts of energy. |
|
|
What do Nitrosomonas and Nitrobacter do? |
Together they carry out nitrification by oxidizing NH3 (ammonia) to NO3- (nitrate).
Nitrosomonas converts NH3 to NO2-.
Nitrobacter converts NO2- to NO3-.
These two bacteria can be used to remove ammonia from waste water.
Nitrification is often followed by denitrification, the conversion of NO3 to N2. |
|
|
What are the two parts that photosynthesis is divided into? |
Light reactions: trap light energy and convert it into chemical energy.
Dark reactions: reduce CO2 to synthesize cell material. |
|
|
Oxygenic photosynthesis |
Oxidizing H2O for electrons to form oxygen.
Oxygenic photosynthesis is carried out by eukaryotes and cyanobacteria.
Cyanobacteria and Green sulfur bacteria. |
|
|
Anoxygenic photosynthesis |
When electrons are taken from a different source other than water .
Other than Cyanobacteria, all other bacteria carry out anoxygenic photosynthesis.
Requires the use of different kinds of pigments.
Green nonsulfur bacteria, Purple sulfur bacteria, Purple nonsulfur bacteria, and Prochloron.
|
|
|
Bacteriochlorophylls |
Major light absorbing pigment in purple and green photosynthetic bacteria. |
|
|
Bacteria Prochlorococcus |
Photosynthetic organism found in aquatic ocean environments.
One of the smallest known photosynthetic organisms whose genome is about 2000 genes.
Ocean samples can contain 100,000 cells/mL. |
|
|
What are the uses of accessory pigments? |
1). Transfer light energy to chlorophylls
2). Absorb different wavelengths of light than chlorophylls, helping to increase the range of wavelengths usable by an organism.
3). Quench toxic forms of oxygen.
Ex). Carotenoids and Phycobiliproteins. |
|
|
Photosystems |
An organization of chlorophylls and accessory pigments to optimize light-harvest.
Photosystems are embedded in thylakoids. |
|
|
Archaeorhodopsin |
Pigment used in Archaea to execute photosynthesis. |
|
|
Bacteriorhodopsin |
Pigment used by Bacteria to execute photosynthesis that is similar to archeorhodopsin. |
|
|
Rhodopsin |
Pigment protein (made up of pigment and protein).
7 transmembrane helicies (similar to G-proteins).
Contains the pigment retinal. |
|
|
Retinal |
Pigment found in rhodopsin.
When the pigment absorbs light, the pigment changes conformation. When rhodopsin changes conformation, the protein portion of rhodopsin changes conformation.
The change in protein conformation allows for protons to be pumped through the plasma membrane. |
|
|
Precursor metabolites |
Molecules that can be utilized in anabolism to construct many different molecules.
Ex). Fructose-6-Phosphate, pyruvate, oxaloacetate |
|
|
RUBISCO |
Enzyme that catalyzes the reaction of ribulose 1,5-bisphosphate with H2O and CO2 into 2 molecules of 3-phosphoglycerate.
This reaction typically occurs in carboxysomes.
This stage of the Calvin cycle is known as the carboxylation phase. |
|
|
Gluconeogenesis |
When glucose is produced fro noncarbohydrate substances.
Animals, plants, fungi, and microbes are capable of executing gluconeogenesis.
Requires the input of ATP and GTP.
6 enzymes are shared between glycolysis and gluconeogenesis, but 4 enzymes are unique to gluconeogenesis. |

