![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
22 Cards in this Set
- Front
- Back
|
What are degradation signals?
|
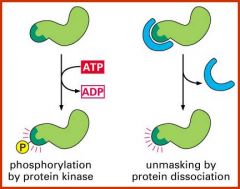
Signals which are quickly created or inmasked to target a protein for destruction eg. through phosphorylation of protein kinase or protein dissociation.
|
|
|
Give an example of an intermediary between degradation signals and degradation machinery.
|
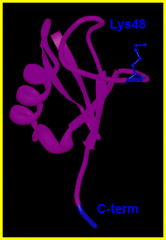
Ubiquitin. Chains of it attach to the cytosolic proteins by attaching the C-terminus of a new molecule to Lys48 of the previous one.
Monoubiquitinylation signals the degradation of integral membran proteins. |
|
|
How does proteosomal degradation work?
|
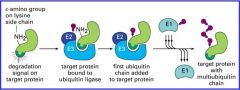
Cytosolic proteins designed for degradation are polyubiquitinylated by ubiquitin ligases once their degradation signal is unmasked.
|
|
|
What are the steps involved in regulated protein degradation II?
|
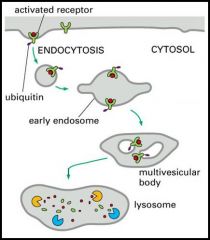
1. For inactivation, surface receptors are ubiquitinylated
2. Monoubiquitinylation of receptors induces their endocytosis 3. Endocytosed ubiquitinylated receptors are sequestered into internal vesicles of a compartment called the multivesicular body 4. Internal vesicles and their content are degraded in the lysosome. |
|
|
How is glycogen phosphorylase regulated?
|
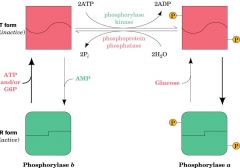
Regulated by various metabolites including adrenaline, glucagon and insulin to either be in active (R) or inactive (T) form. Kinase mediated phosphorylation also regulates allosteric regulation;
dephosphorylised = phosphorylase b phosphorylised = phosphorylase a |
|
|
Give an example of a second type of conformational change involved in regulation.
|
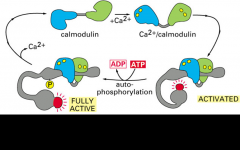
Calmodulin is activated by calcium when there is an increase in calcium concentration. Calmodulin undergoes a large conformational change, activating many downstream targets.
|
|
|
Give a third example of a type of conformational change involved in regulation
|
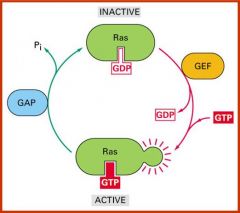
GTPases change conformation when hydrolysing GTP. Two switch regions change conformation, allowing GTPase to bind and modulate specific targets in the GTP bound form.
GTPases are regulated by GEF and GAP |
|
|
What side chains can be modified by phosphorylation?
|
Serine, threonine or tyrosine side chains.
|
|
|
What two things can phosphorylation do?
|
1. Promote/inhibit the function of a protein
2. Influence enzymatic activity, protein conformation or protein interactions |
|
|
Name two kinases central for metabolism
|
mTOR
AMPK |
|
|
Give examples of AGC (protein kinase A, G C)
|
PKA, Akt/PKB, PKC, PKG
|
|
|
Give two examples o calmodulin activated (CaM) kinases
|
CaMII
PKD (lipid activation (DAG) |
|
|
What proteins are involved in partitioning between the cytosol and the membrane?
|
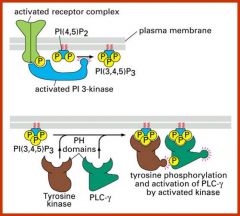
Phosphoinositides. These can be interconverted by kniases and phosphatases which allows the speciic co-localization of signalling components, which results in their activation
|
|
|
What proteins are involved in movement between intracellular compartments?
|
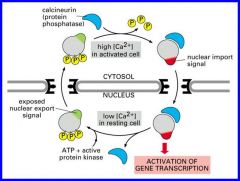
Transcriptional activators- shuttle between nucleus and cytosol by exposing nuclear import and export signals.
|
|
|
How do metabolite transporters and receptors avoid active participation in signalling at the plasma membrane?
|
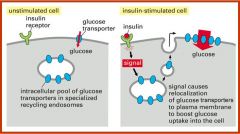
Use the endpcytic pathway
|
|
|
What are transient protein complexes?
|
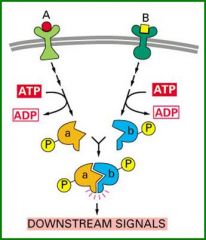
Generated for a given reaction and then dissociated to stop that reaction.
They need at least one component to change its binding properties prior to binding and revert to the inactive state to dissolve the complex. |
|
|
What is signal integration?
|
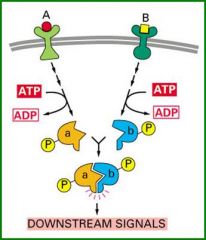
When signalling from two pathways are integrated by two of their cascade components interacting and transferring the signal jointly.
|
|
|
Explain signal handover in the context of G proteins
|
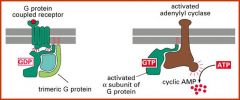
Ga subunit of trimeric G protein is activated by GPCR. It then receives GTP which allows it to bind to adenylyl cyclase. Upon GTP hydrolysis the transient Ga-adenylyl cyclase interaction is lost.
|
|
|
Give examples of protein domains which have evolved to bind to particular motifs
|
SH2- binds P-Tyr
SH3- binds proline rich region PH- binds P14, 5P2 |
|
|
What are the key features of an SH2 domain?
|
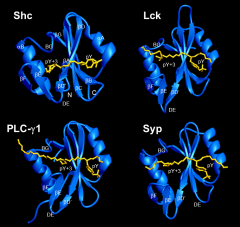
Amino acids around pTyr provide a specificity signature:
Shc = Leu or Val at 3rd position Lck = Ile at 3rd position PLC-yl = needs hydrophobic residue at 2nd position Syp = needs specific residues up to 5 aas away from pTyr |
|
|
Give two examples of pathways in which scaffolds are used.
|
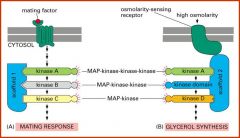
Cascade for mating response and glycerol synthesis; both start with same kinase but scaffolds direct signals to different targets.
|
|
|
What role do scaffolds play in protein interaction?
|
1. Direct cascades to different targets
2. Bring components of signal pathway together to strengthen weak interaction and allow signal propagation |

