![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
355 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What happens during fasting? |
Pancreatic alpha cells release glucagon, triggered by a fall in blood glucose (skeletal muscle does not react to glucagon) The liver no longer uses glucose as a fuel source Muscle switches to FA and ketone bodies, the brain starts to use ketone bodies RBC, Rena medulla are the only once’s using glucose still Protein is used from muscle as a last resort during starvation |
|
|
|
During feeding, what happens to lipids? |
TAGs are absorbed, via the lymph and enter the main circulation Taken up by adipose tissue and stored Used by muscle as an energy source |
|
|
|
What happens during the early fasting state? |
During initial unfed state of fasting, liver glycogen is broken down and glucose is released into circulation Glucose is used by tissues (brain, RBC, muscle) The alanine cycle becomes important (alanine generates fromcamination is pyruvate) the alanine is taken up by the liver and deaminated, nitrogen excreted as urea and the pyruvate is conveyed to glucose by gluconeogenesis The Cori cycle operates |
|
|
|
What happens during the early fasting state? |
During initial unfed state of fasting, liver glycogen is broken down and glucose is released into circulation Glucose is used by tissues (brain, RBC, muscle) The alanine cycle becomes important (alanine generates fromcamination is pyruvate) the alanine is taken up by the liver and deaminated, nitrogen excreted as urea and the pyruvate is conveyed to glucose by gluconeogenesis The Cori cycle operates |
|
|
|
During feeding, what happens to glucose? |
Taken up by liver and stored as glycogen Can be metabolised to pyruvate en route to fat synthesis TAGs synthesised in the liver are carried in the blood in the form VLDLs Glucose continues into the circulation Used by the brain and testes (solely glucose users) and RBC, renal medulla Insulin triggers insertion of GLUT4 transporters to increase uptake capacity Glucose is also taken up by adipocytes for conversion into fat |
|
|
|
What happens to the energy requirements of skeletal muscle during aerobic exercise (long term)? |
As there is not enough stored glycogen in skeletal muscle form long term exercise... Increase in lipolysis - FA used correctly as fuel by muscle or as ketone bodies Progressive switch over to preferential fatty acid oxidation by muscle |
|
|
|
During feeding, what happens to protein? |
Taken up by liver Used by liver for protein synthesis Deamination to give alpha-ketoacid (ultimately pyruvate) and ammonia Pyruvate used for fat and glucose/glycogen synthesis; ammonia excreted via urea cycle Protein is then passes into main circulation for protein synthesis in all tissues |
|
|
|
What is Graves’ disease? |
Autoimmune- autoantibodies Bind and activate thyroid stimulating hormone receptor, leading to continuous thyroid hormone synthesis |
|
|
|
What is the most common form of hyperthyroidism? |
Graves’ disease |
|
|
|
What happens to the energy requirements of skeletal muscle during aerobic exercise (short to moderate term)? |
Glucose from muscle glycogen is the main source of energy Glucose uptake from the blood is also increased by non-insulin-dependent insertion of GLUT4 into the muscle cell plasma membrane Glucose is released from liver glycogenolysis Muscle also increased oxidation of branched-chain amino acids. Ammonium production and alanine release |
|
|
|
What happens to the energy requirements of skeletal muscle during aerobic exercise (long term)? |
As there is not enough stored glycogen in skeletal muscle form long term exercise... Increase in lipolysis - FA used correctly as fuel by muscle or as ketone bodies Progressive switch over to preferential fatty acid oxidation by muscle |
|
|
|
During feeding, what happens to glucose, amino acids and lipids? |
Glucose and amino acids are taken up by the liver by the portal vein before entering general circulation. A rise in blood glucose triggers insulin release Lipids are absorbed via the lymph system which drains into the vena cava, transported in lymph by chylomicrons |
|
|
|
What is the thyroid hormone important in? |
Growth in children Development of fetal brain Cardiovascular system (HR, CO) Reproductive system (infertility) |
|
|
|
What can cause hypothyroidism? |
Iodine deficiency Primary thyroid disease |
|
|
|
During feeding, what happens to glucose, amino acids and lipids? |
Glucose and amino acids are taken up by the liver by the portal vein before entering general circulation. A rise in blood glucose triggers insulin release Lipids are absorbed via the lymph system which drains into the vena cava, transported in lymph by chylomicrons |
|
|
|
What metabolic activities does thyroid hormone stimulate? |
Lipid metabolism ( increases fat metabolism, FA oxidation) Carbohydrates metabolism (increases gluconeogenesis and glycogenolysis) |
|
|
|
How is glycogen synthesis controlled in the liver and muscle? |
Intrinsic regulation Extrinsic regulation |
|
|
|
Where does the glucose-fatty acid cycle occur? |
Adipose tissue and muscle |
|
|
|
How long will the fat stores last |
3 months |
|
|
|
During feeding, what happens to protein? |
Taken up by liver Used by liver for protein synthesis Deamination to give alpha-ketoacid (ultimately pyruvate) and ammonia Pyruvate used for fat and glucose/glycogen synthesis; ammonia excreted via urea cycle Protein is then passes into main circulation for protein synthesis in all tissues |
|
|
|
What are the symptoms of hypothyroidism? |
Goiter Lethargy, fatigue, weakness, hair loss, reproductive failure |
|
|
|
What happens to the energy requirements of skeletal muscle during aerobic exercise (short to moderate term)? |
Glucose from muscle glycogen is the main source of energy Glucose uptake from the blood is also increased by non-insulin-dependent insertion of GLUT4 into the muscle cell plasma membrane Glucose is released from liver glycogenolysis Muscle also increased oxidation of branched-chain amino acids. Ammonium production and alanine release |
|
|
|
What happens to the energy requirements of skeletal muscle during anaerobic exercise? |
Energy for muscle contraction comes from intramuscular stores (glycogen and phosphocreatine) Due to lack of oxygen. Muscle cells are glycolytic, producing a lot of lactic acids which cause cramps and weakening. This drops intracellular pH which inhibits glycolysis. And limits lactate formation |
|
|
|
What is the most common form of hyperthyroidism? |
Graves’ disease |
|
|
|
Can the brain use anaerobic glycolysis? |
No |
|
|
|
When do we convert free amino acids to a common metabolic pool? |
During catabolism: starvation And anabolism: excess protein intake |
|
|
|
During feeding, what happens to glucose? |
Taken up by liver and stored as glycogen Can be metabolised to pyruvate en route to fat synthesis TAGs synthesised in the liver are carried in the blood in the form VLDLs Glucose continues into the circulation Used by the brain and testes (solely glucose users) and RBC, renal medulla Insulin triggers insertion of GLUT4 transporters to increase uptake capacity Glucose is also taken up by adipocytes for conversion into fat |
|
|
|
What fuel does the heart prefer? |
NEFAs and endogenous triglycerides |
|
|
|
What happens during fasting? |
Pancreatic alpha cells release glucagon, triggered by a fall in blood glucose (skeletal muscle does not react to glucagon) The liver no longer uses glucose as a fuel source Muscle switches to FA and ketone bodies, the brain starts to use ketone bodies RBC, Rena medulla are the only once’s using glucose still Protein is used from muscle as a last resort during starvation |
|
|
|
What is Graves’ disease? |
Autoimmune- autoantibodies against |
|
|
|
During feeding, what happens to lipids? |
TAGs are absorbed, via the lymph and enter the main circulation Taken up by adipose tissue and stored Used by muscle as an energy source |
|
|
|
What happens to the energy requirements of skeletal muscle during anaerobic exercise? |
Energy for muscle contraction comes from intramuscular stores (glycogen and phosphocreatine) Due to lack of oxygen. Muscle cells are glycolytic, producing a lot of lactic acids which cause cramps and weakening. This drops intracellular pH which inhibits glycolysis. And limits lactate formation |
|
|
|
What does thyroid hormone do? |
Stimulate diverse metabolic activities leading to an increase in basal metabolic rates |
|
|
|
What happens to levels of plasma NEFA levels under different metabolic conditions? |
Normal - sub mM Starvation - 1mM |
|
|
|
What regulates carbohydrate response to nutritional status? |
Pyruvate kinase, pyruvate dehydrogenase, FA synthesis, insulin |
|
|
|
What is leptin deficiency associated with? |
Intense hunger and obesity |
|
|
|
What does leptin do? |
Acts through hypothalamic receptors and a complex neuroendocrine system to reduce appetite |
|
|
|
What is leptin? |
Peptide hormone secreted by adipocytes in response to the amount of fat stored. Increased fat strokes leads to increases in circulating leptin concentrations |
|
|
|
What fuel does the heart prefer? |
NEFAs and endogenous triglycerides |
|
|
|
What happens during later fasting state/ starvation? |
Liver is already gluconeogenic but also becomes ketogenic and peoteolytic Ketone bodies formed from FA released by lipolysis in adipose FA -> fuel in muscle Ketone bodies -> fuel in brain and muscle Glycerol from TAG breakdown is used by liver for gluconeogenesis Protein hydrolysis takes place in the muscle and alanine and glutamine are release Alanine -> alanine cycle Glutamine -> metabolised by enterocytes |
|
|
|
What is the thyroid hormone important in? |
Growth in children Development of fetal brain Cardiovascular system (HR, CO) Reproductive system (infertility) |
|
|
|
What metabolic activities does thyroid hormone stimulate? |
Lipid metabolism ( increases fat metabolism, FA oxidation) Carbohydrates metabolism (increases gluconeogenesis and glycogenolysis) |
|
|
|
What can cause hypothyroidism? |
Iodine deficiency Primary thyroid disease |
|
|
|
What are the symptoms of hypothyroidism? |
Goiter Lethargy, fatigue, weakness, hair loss, reproductive failure |
|
|
|
What happens during later fasting state/ starvation? |
Liver is already gluconeogenic but also becomes ketogenic and peoteolytic Ketone bodies formed from FA released by lipolysis in adipose FA -> fuel in muscle Ketone bodies -> fuel in brain and muscle Glycerol from TAG breakdown is used by liver for gluconeogenesis Protein hydrolysis takes place in the muscle and alanine and glutamine are release Alanine -> alanine cycle Glutamine -> metabolised by enterocytes |
|
|
|
What regulates insulin response elements to nutritional status? |
Hexokinase, acetyl-CoA carboxylase, G-6-P, PPARS |
|
|
|
What is leptin? |
Peptide hormone secreted by adipocytes in response to the amount of fat stored. Increased fat strokes leads to increases in circulating leptin concentrations |
|
|
|
What does leptin do? |
Acts through hypothalamic receptors and a complex neuroendocrine system to reduce appetite |
|
|
|
What does thyroid hormone do? |
Stimulate diverse metabolic activities leading to an increase in basal metabolic rates |
|
|
|
What regulates carbohydrate response to nutritional status? |
Pyruvate kinase, pyruvate dehydrogenase, FA synthesis, insulin |
|
|
|
What is leptin deficiency associated with? |
Intense hunger and obesity |
|
|
|
Give an example of the concept of incoupling |
Thermogenesis in brown tissue - heat production |
|
|
|
Where does the TCA cycle take place? |
Mitochondrial matrix |
|
|
|
What is the electron transport chain? (Structure) |
4 enzyme complexes ending with transfer of electrons to molecular oxygen |
|
|
|
Where does the TCA cycle take place? |
Mitochondrial matrix |
|
|
|
What is the electron transport chain? (Structure) |
4 enzyme complexes ending with transfer of electrons to molecular oxygen |
|
|
|
What regulates insulin response elements to nutritional status? |
Hexokinase, acetyl-CoA carboxylase, G-6-P, PPARS |
|
|
|
How is acetyl-CoA formed from pyruvate? |
Link reaction by pyruvate dehydrogenase reaction |
|
|
|
Metabolic control can either be intrinsic or extrinsic, give a meaning for each plus example |
Intrinsic: bought about by changes in intracellular levels of an allosteric regulator of an enzyme Extrinsic: bought about by signals originating outside the cell, e.g. hormones, small changes in plasma hormone levels have large effects on cell function due to amplification cascade |
|
|
|
What major metabolic pathways occurs in the Endoplasmic reticulum? |
Smooth ER: elongation pc FA chains over C16 during biosynthesis of lipids. Final step in the gluconeogenic pathway in the liver and renal cortex. Site of enzyme glucose-6-phosphatase Rough ER: protein synthesis on ribosomes |
|
|
|
What enters the TCA cycle |
acetyl-CoA |
|
|
|
What does the electron transport do? |
Converts the electrons stored as reduced intermediates NADH and FADH2 into a proton motive force across the internal mitochondria membrane |
|
|
|
Where does the TCA cycle take place? |
Mitochondrial matrix |
|
|
|
What enters the TCA cycle |
acetyl-CoA |
|
|
|
What is metabolism? |
All the chemical reactions that take place in the living organism |
|
|
|
What are Anabolic reactions |
Takes in energy, used for storage and biosynthesis e.g monosaccharides from diet to glycogen |
|
|
|
What is Catabolic reactions |
Releases energy. Degradation of compounds. E.g glycogen to glucose |
|
|
|
What is oxidation (in general biology) |
Extraction of electrons from metabolic fuels |
|
|
|
What are the products of complete oxidation? |
Production of water, carbon dioxide |
|
|
|
What are the products of partial oxidation? |
Carbon monoxide and heat formed |
|
|
|
Give examples of oxidised and their reduced forms for proton/electron carriers |
NAD+ (oxidised) —> NADH (reduced)
FAD (oxidised —> FADH2 (reduced) |
|
|
|
In cellular respiration, where are NADH and FADH2 deoxidised? |
Electron transport chain |
|
|
|
How is most energy in the form of? |
ATP |
|
|
|
What is the general process for generating ATP |
Cellular respiration |
|
|
|
How does ATP transfer it’s energy? |
Releasing Pi |
|
|
|
For glucose, what is the general equation for respiration |
C6H12O6 + 6O2 —> 6CO2 + 6H2O |
|
|
|
What is the respiratory quotient of the general glucose reaction? |
6CO2/6O2 = 1 |
|
|
|
What is the general equation for lipids? |
C15H31COOH (palmitic acid) + 23O2 —> 16CO2 + 16H2O |
|
|
|
What is the respiratory quotient for lipid respiration equation? |
16CO2/23O2 = 0.7 |
|
|
|
Why is the respiratory quotient lower for lipids than glucose? |
Lipids are more reduced than glucose so need more oxygen to fully oxidise |
|
|
|
What controls ATP synthesis |
ADP AMP ADP - control at mitochondrial level through respiratory control. There must be ADP in the ETC to convert to ATP or it will not run AMP - important intracellular signal. Rise in AMP activates the glycolysis pathway via PFK enzyme and stimulates ATP production |
|
|
|
What are the general concentrations of ATP, ADP AND AMP? |
ATP - high ADP - low AMP - very low |
|
|
|
What is the daily turnover of ATP in the average 70kg man? |
40kg |
|
|
|
What major metabolic pathways occurs in the mitochondria |
ATP production in all cells except erythrocytes Krebs cycle, ETC, oxidative phosphorylation, beta-oxidation, synthesis of urea, haem synthesis |
|
|
|
What major metabolic pathways occurs in the Endoplasmic reticulum? |
Smooth ER: elongation of FA chains over C16 during biosynthesis of lipids. Final step in the gluconeogenic pathway in the liver and renal cortex. Site of enzyme glucose-6-phosphatase Rough ER: protein synthesis on ribosomes |
|
|
|
What major metabolic pathways occur in the Golgi apparatus? |
Modification of proteins, protein sorting for appropriate trafficking, manufacturing of new lipid membranes |
|
|
|
What major metabolic pathway occurs in the lysosomes? |
Intracellular digestion of extracellular and intracellular components |
|
|
|
What major metabolic pathway occurs in the peroxisomes? |
Peroxisomal enzymes play an essential role in lipid breakdown |
|
|
|
Metabolic control can either be intrinsic or extrinsic, give a meaning for each plus example |
Intrinsic: bought about by changes in intracellular levels of an allosteric regulator of an enzyme Extrinsic: bought about by signals originating outside the cell, e.g. hormones, small changes in plasma hormone levels have large effects on cell function due to amplification cascade |
|
|
|
Give 2 examples of short term controls of metabolism |
1. Allosteric (milliseconds): bonding of molecules to enzymes affecting their catalytic rate by affecting a change in their properties of another site on the same protein. E.g. positive and negative feedback effects exerted by metabolic intermediates
2. Covalent modification (secs to mins): enzyme catalysed alterations of synthesised proteins and includes addition or removal of chemical groups. E.g. covalent modifications like phosphorylation (ATP-> ADP) |
|
|
|
An example of long term controls of metabolism |
Changes in enzyme protein levels via TF (hours to days) |
|
|
|
What is the convention of glucose to lactate called? |
Cori cycle |
|
|
|
What process is lactate produced by |
Anaerobic glycolysis |
|
|
|
Outline the Cori cycle |
1. In the muscle during exercise, glycogen is converted by anaerobic glycolysis to G-6-P, then pyruvic acid and to lactic acid. 2. Lactic acid travels in the blood to the liver 3. Lactic acidosis -> pyruvic acid -> G-6-P -> glycogen 3. Or, G-6-P removes from liver as glucose in the blood to the muscles |
|
|
|
What aids metabolism at a cellular level? (In terms of appropriate location) |
1. Delivery (anatomy, functioning circulation) 2. Transmembrane movement (membrane transporters) of substrates and enzyme regulation. |
|
|
|
What do oxidation reduction reactions involve chemically? |
Catabolic (breakdown) reactions involve oxidation Anabolic (synthesis) reactions involve reduction |
|
|
|
Name the three biological intermediates (oxidation/reduction) |
NAD FAD NADP |
|
|
|
Outline the TCA cycle |
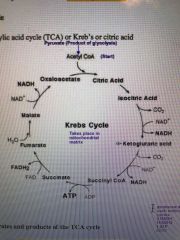
Pyruvate starts |
|
|
|
What is the overall products of TCA cycle? (Starting from acetyl CoA, in one turn) |
2CO2, 3NADH and 1FADH2 |
|
|
|
What enters the TCA cycle |
acetyl-CoA |
|
|
|
How many carbons does pyruvate have? |
3 |
|
|
|
How many carbons does acetyl-CoA have? |
2 |
|
|
|
How is acetyl-CoA formed from pyruvate? |
Link reaction by pyruvate dehydrogenase reaction |
|
|
|
Where can acetyl- CoA come from, before entering the TCA cycle? |
Pyruvate (glycolysis) FA breakdown Carbon skeleton of Aa |
|
|
|
Where does the TCA cycle take place? |
Mitochondrial matrix |
|
|
|
What is the electron transport chain? (Structure) |
4 enzyme complexes ending with transfer of electrons to molecular oxygen |
|
|
|
What does the electron transport do? |
Converts the electrons stored as reduced intermediates NADH and FADH2 into a proton motive force across the internal mitochondria membrane |
|
|
|
What transition metals are involved with the electron transport chain? And where? |
Iron in haem, Fe-S and Cu2+ embedded in proteins ( I, III, IV respectively) |
|
|
|
Draw the electron transport chain |
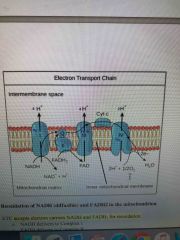
Back (Definition) |
|
|
|
How many H+ are extruded from each complex in the ETC? |
Complex 1: 4H+ Complex 2: 4H+ Complex 4: 2H+ |
|
|
|
For each NADH AND FADH2 oxidised, how many H+ are extruded in the ETC |
NADH - 10H+ FADH2 - 6H+ |
|
|
|
What is the ultimate electron acceptor in the ETC |
Oxygen |
|
|
|
Which complexes do NADH and FADH deliver, in the ETC? |
NADH - Complex I FADH - Complex II |
|
|
|
What is oxidative phosphorylation? |
Passage of electrons along the respiratory chain associated with pumping of protons out of the mitchondrial matrix, the H+ returns through ion channels which is part of the enzyme ATP synthase creating ATP |
|
|
|
How many protons are required to produce 1 ATP during oxidative phosphorylation? |
3H+ to make 1 ATP However requires another H+ to generate pump so actually is 4H+ for 1 ATP |
|
|
|
Explain the ATP synthase mechanics? How it works? |
Movement of protons through F0 subunit (protein channel) indices the F1 subunit (catalytic site for ATP st grade) to physically rotate - proposed that protons drive the binding sites through their different states of loose (ADP+Pi), tight (ADP+Pi) and ATP release, therefore it takes 3 H+ to make one ATP (however needs to transport as well so actually 4H+ protons per ATP) |
|
|
|
Give an example of the concept of uncoupling |
Thermogenesis in brown tissue - heat production |
|
|
|
Explain thermogenesis in brown tissue |
In adipose tissue there is an uncoupling protein that allows the PMF to be dissipates without marking ATP. This is important in babies who cannot shiver to generate heat |
Concept of uncoupling |
|
|
Give an example of an uncoupling agent? |
Dinitrophenol |
|
|
|
What does dinitrophenol do? |
Collapse the PMF inhibit ATP formation. Tend to be weak lipophilic acids that carry protons across the IMM |
|
|
|
What locations are glucose stored |
Liver - to maintain blood glucose during short periods of fasting (24h storage) Skeletal muscle- can only be used by muscle itself during exercose |
|
|
|
What is the average concentration of glucose in the blood |
5mM in 3L blood |
|
|
|
What is the major store of lipids |
Adipose tissue |
|
|
|
How long will the fat stores last |
3 months |
|
|
|
When is protein used as an energy source? |
In extreme starvation and only when all other stores have been exhausted |
|
|
|
Where is the protein used during energy requirements |
Skeletal muscle Loss of protein from heart, kidney, and liver compromise function |
|
|
|
What percentage of our daily energy intake is carbohydrates? |
35-45% |
|
|
|
How is fat absorbed from the diet? |
Triacylglycerides hydrolysed by pancreatic lipase to free FA and monoglycerides Emulsification breaks down fat globules into smaller emulsion droplets These are coated in bile salts and incorporated to form micelles Travel to enter pure cell wall which via membrane transport proteins Inside enterocyte, packages into chylomicrons (vehicle to carry fat) Chylomicrons pass into lymph system and drained into vena cava |
|
|
|
What percentage of our daily energy intake is fat? |
40-50% |
|
|
|
What percentage of our daily energy intake is protein? |
7-10% |
|
|
|
What is the energy yield of carbohydrate, fat and protein per day? |
Carbohydrates: 4kcal/g Fat: 9kcal/g Protein: 4kcal/g |
|
|
|
For a diet to work, how should energy intake be related to energy exposure? |
Energy intake must be less than energy exposure |
|
|
|
What determines resting energy expenditure? |
Basal metabolic rate |
|
|
|
What determines basal metabolic rate? |
Lean body mass and thyroid activity |
|
|
|
Daily energy exposure can be expressed as a ratio to resting metabolic rate (the physical activity level - PAL). What is the PAL for sedentary people, highly active people and endurance athletes? |
Sedentary people - PAL = 1.4 Highly active people - PAL = 2.5 Endurance athletes - PAL = 3-4 |
|
|
|
What are the advantages and disadvantages of fat as a metabolic fuel? |
Ads: Compact, low weight, double the energy per gram than glycogen. Low hydration level Dis: slow to release |
|
|
|
How is fat absorbed from the diet into the blood? |
Triacylglycerides hydrolysed by pancreatic lipase to free FA and monoglycerides Emulsification breaks down fat globules into smaller emulsion droplets These are coated in bile salts and incorporated to form micelles Travel to enter pure cell wall which via membrane transport proteins Inside enterocyte, packages into chylomicrons as triacylglycerides again (vehicle to carry fat) Chylomicrons pass into lymph system and drained into vena cava |
|
|
|
What does lipoprotein lipase do, and on which tissues can they be found? |
Found membrane bound on adipose and muscle They break the triacylglycerides into free FA and monoacylglycerides |
|
|
|
How are the products (FA and monoacylglycerides) taken up by tissues? |
Diffusion and mediates transport |
|
|
|
What is the carnitine shuttle? |
Responsible for transferring long-chain FA across the barrier of the inner mitochondrial membrane to gain access to the enzymes of beta-oxidation |
|
|
|
How does the carnitine shuttle work? |
Long chain FA are conjugated to carnitine to for acyl carnitine (catalysed by CAT-1) Acyl carnitine crosses the IMM in a specific carrier - acyl carnitine translocase (strongly inhibited by malonyl CoA) Acyl carnitine is reconverted to acyl-CoA and free carnitine in the mitochondrial matrix (catalysed by CAT-II) |
|
|
|
What are NEFAs? |
Any FREE FA which is therefore not esterified with glycerol |
|
|
|
What process happens to the acyl-CoA in the mitochondrial matrix |
Beta-oxidation |
|
|
|
What is the process of beta-oxidation? |
Four step cyclic reaction that removes C2 unit in the form of acetyl-CoA which then enters the TCA cycle |
|
|
|
What is the cellular mechanism that these hormones regulate lipase activity? |
Hormones bind to G-protein coupled receptors Activates adenylate Cyclase Raises cAMP and activating PKA PKA phosphorylates triacylglycerol lipase, activating it to breakdown triacylglycerides |
|
|
|
What happens to levels of plasma NEFA levels under different metabolic conditions (normal and starvation)? |
Normal - sub mM Starvation - 1mM |
|
|
|
What are the numbers of ketone bodies present in the blood during different metabolic conditions (normal, starvation and T1DM)? |
Normal (fed): virtually absent Starvation: 5mM T1DM- uncontrolled leading to metabolic acidosis |
|
|
|
What is the pathway of FA oxidation into fatty acyl CoA? |
FA in the blood stream then Enter cell, FA are oxidised in the mitochondrial matrix On diffusing across the membrane, the hydrophobic fatty acids associate with a cytoplasmic binding protein (goes to mito matrix). Medium chain (C8-C10) can cross the IMM directly Long chains need to be activated before they can cross IMM -> a CoA group is joined by a thioester linkage to the carboxyl group of the FA (driven by ATP, catalysed by acyl-CoA synthase) produces fatty acyl-CoA and H2O |
|
|
|
How are the products (FA and monoacylglycerides) takes up by tissues? |
Diffusion and mediates transport |
|
|
|
What happens to the extra sugars when glycogen stores have been fully replenished? |
Liver switches to fat biosynthesis |
|
|
|
Where and how are FA made in the liver? |
Cytosol by large enzymes known as FA synthase |
|
|
|
What process happens to the acyl-CoA in the mitochondrial matrix |
Beta-oxidation to become acetyl-CoA |
|
|
|
During starvation, how does the liver maintain blood glucose levels? |
Gluconeogenesis Oxoacetate from the TCA cycle (starting substrate of gluconeogenesis) note: the removal of oxoacetate prevents acetyl-CoA from entering TCA The build up of acetyl-CoA leads to a greatly increased rate of formation of ketone bodies in the mitochondria (ketogenesis) |
|
|
|
What are major ketone bodies? |
Acetoacetate and beta-hydroxybutyrate |
|
|
|
Can the liver metabolise ketone bodies? |
No - lacks enzyme beta-ketoacyl-CoA transferase |
|
|
|
What are recipient tissues of ketone bodies? |
Heart, brain, renal cortex, adrenal gland |
|
|
|
Why does the brain switch to using ketone bodies? |
To reduce gluconeogenesis load on the body, preserving protein (muscle) from breakdown |
|
|
|
Why are ketone bodies a gold source of energy during starvation? |
Efficient metabolic resources (produced loads of ATP). Also provides a survival advantage to the tissues that receive them from the liver |
|
|
|
What metabolic state does insulin signal? |
Signals well fed state |
|
|
|
What happens to the FA and monoacylglycerides once they have been transported into the tissues? |
reassemble into triacylglycerides |
|
|
|
When does glycogen and adrenaline signal? |
When there is a need for energy release from fat |
|
|
|
When does thyroxine signal? |
Long term signal of growth and development |
|
|
|
What effect does insulin have on the synthesis, breakdowns uptake and release of FA and ketogenesis? |
Turns on lipid synthesis - activates acetyl-CoA carboxylate by dephosphorylation Increases the amount of lipoprotein lipase on the endothelial cells in adipose tissue - increases the breakdown of circulating TAGs and thus their uptake and storage in adipocytes |
|
|
|
What effect does glucagon/adrenaline have on the synthesis, breakdowns uptake and release of FA and ketogenesis? |
Activate PKA (protein kinase A) via cAMP which: 1. Inhibits fat synthesis by phosphorylating acetyl-CoA carboxylate and thus inactivating it 2. Promotes breakdown of TAGs in adipose tissue and release of FA by activating lipases 3. Reduces the amount of lipoprotein lipase so that circulating TAGs are available for other tissues |
|
|
|
What effect does thyoxine have on the synthesis, breakdowns uptake and release of FA and ketogenesis? |
Increases the oxidative metabolism of both carbohydrates and fat |
|
|
|
What happens when fats are required for use? |
Lipolysis is used to break down stores triacylglycerides Released non-esterified fatty acids (NEFAs) leave the cell and are transported in the blood bound to albumin to peripheral tissues |
|
|
|
W |
Any FREE FA which is therefore not esterified with glycerol |
|
|
|
What physical food intake state does glucagon and adrenaline signal? |
When there is a need for energy release from fat |
|
|
|
What hormones regulate lipase activity? |
Glucagon, adrenaline, noradrenaline, ACTH |
|
|
|
What is the mechanism that these hormones regulate lipase activity? |
Hormones bind to G-protein coupled receptors Activates adenylate Cyclase Raises cAMP and activating PKA PKA phosphorylates triacylglycerol lipase, activating it to breakdown triacylglycerides |
|
|
|
What effect does glucagon/adrenaline have on the synthesis, breakdowns uptake and release of FA and ketogenesis? |
Activate PKA (protein kinase A) via cAMP which: 1. Inhibits fat synthesis by phosphorylating acetyl-CoA carboxylate and thus inactivating it 2. Promotes breakdown of TAGs in adipose tissue and release of FA by activating lipases 3. Reduces the amount of lipoprotein lipase so that circulating TAGs are available for other tissues |
|
|
|
What are the numbers of ketone bodies present in the blood during different metabolic conditions? |
Normal (fed): virtually absent Starvation: 5mM T1DM- uncontrolled leading to metabolic acidosis |
|
|
|
What are ketone bodies signals for? |
Signals availability of energy substrate High levels of acetoacetate acts as a signal for abundantly available acetyl groups, I.e. inhibits further breakdown of fat in adipose tissue |
|
|
|
Discuss ketone bodies presence in diabetes? |
Lack of insulin secretion means that the liver does not absorb glucose and so the lack of hepatic carbohydrate leads to ketogenesis Ketone bodies are acidic and their accumulation and the ensuing metabolic acidosis can be severe enough to impair CNS function Cancsmell acetoacetate on the breath as acetone |
|
|
|
What is cholesterol used for in the body? |
Key component of membranes (increases membrane fluidity) precursor of steroid hormones, precursor of vitamin D |
|
|
|
What is cholesterol synthesised from? |
Acetyl-CoA |
|
|
|
What is the rate limiting step in cholesterol synthesis? |
Reduction of HMG-CoA to mevalonate by HMC-CoA reductase |
|
|
|
What are the steps of cholesterol synthesis? |
Acetyl-CoA-> HMG-CoA-> mevalonate -> farnesyl-pyrophosphate -> squalene-> cholesterol |
|
|
|
What does dietary cholesterol inhibit? |
Hepatic cholesterol synthesis |
|
|
|
What do statins do to reduce cholesterol? |
Competitively inhibit HMG-CoA reductase |
|
|
|
How is cholesterol synthesis associated with an anabolic state? |
HMG-CoA reductase activity is increased by insulin and thyroxine and decreased by glucagon and cortisol |
|
|
|
What are non-esterified FA bound to when circulating in the blood? |
Albumin |
|
|
|
What is the endogenous pathway of lipoprotein metabolism? |
Distribution of triacylglycerol from liver in the very-low-density lipoproteins (VLDL) |
|
|
|
What are lipoproteins? |
Lipid droplets with a hydrophobic lipid core and a surface monolayer |
|
|
|
What is the process of endogenous pathway of lipoprotein metabolism? |
Chylomicrons (VLDL) are substrates for lipoprotein lipase in capillary beds and so deliver RAG from liver to other tissues Cholerasteryl ester rich particles result in 2 possible fates. 1. Taken up by a receptor in the liver which binds to VLDL-specific lipoproteins, delivering cholesteryl water to tissues. 2. Remain in circulation and become LDL particles |
|
|
|
What are LDL particles formed from? |
Depleted VLDL particles |
|
|
|
Where do LDLs go after production? |
Leave circulation through uptakes by LDL receptors and deliver cholesterol to tissues |
|
|
|
What do macrophages do to LDL particles? |
Macrophages have scavenger receptors and so uptake cholesterol, which can be deposited in arterial walls |
|
|
|
What are HDLs involved in? |
Removal of cholesterol from tissues, transporting it to the liver for excretion directly into bile or converted to bile salts for excretion |
|
|
|
What are HDL formed from? |
Apolipoprotein A1, which are secreted from the liver and intestines |
|
|
|
How does HDL acquire cholesterol? |
1. Interact with cells and collect excess cellular cholesterol 2. Acquire the excess surface material released during the lipolysis of TAG-rich lipoproteins by lipoprotein lipase By these two routes the unesterified cholesterol is esterified to a long-chain FA by LCAT enzyme, these mature |
|
|
|
How do TAGs and cholesterol circulate in the blood? |
Circulate in lipoproteins |
|
|
|
Wha |
Lipid droplets with a hydrophobic lipid core and a surface monolayer |
|
|
|
What are the types of lipoproteins? |
High density lipoproteins Very low sensory lipoproteins Low density lipoproteins |
|
|
|
What regulates lipoprotein metabolism in the short term and long term? |
Short term - insulin Long term - transcriptional mechanisms that operate within the cells |
|
|
|
What are elevated levels of LDL-Cholesterol and TAGs in the blood associated with? |
Atherosclerosis risk |
|
|
|
What is atherosclerosis? |
Cholesterol is taken up by macrophages in the arterial wall and deposited |
|
|
|
What are the risk factors for elevated lipid levels? |
Obesity and lack of exercise |
|
|
|
What is the exogenous pathway of lipoprotein metabolism? |
Refers to the distribution of dietary fat (I.e. outside) which is mostly triacylglycerol |
|
|
|
Outline the exogenous pathway of lipoprotein metabolism? |
TAG and cholesterol are absorbed and re-esterified in the cells of the intestinal wall and secreted as chylomicron particles vis lymphatics into circulation Chylomicrons interact with other particles and acquire apolipoprotein CII which makes them substrates for the action of lipoprotein lipase as they pass through capillaries of tissues expressing this enzyme such as adipose tissue and muscle Thus TAG is hydrolysed and particle shrinks resulting in chylomicron remnants Chylomicron remnants are endocytosed and hydrolysed within lysosomes. This releases glycerol and FA in the cell |
|
|
|
What state is glucose stored in? |
Glycogen |
|
|
|
Does the renal medulla require glucose, if so, why? |
Yes. As it is a high energy power |
|
|
|
What is the pathway called of glucose metabolism? |
Glycolysis |
|
|
|
What happens in anaerobic conditions in generating ATP? |
Glycolysis yields lactate. Which can be sent to the liver and converted back to glucose via Cori cycle Only short term energy peridos |
|
|
|
What is the general reaction of glycolysis? |
Splits a 6C into two 3C pyruvate molecules |
|
|
|
Under aerobic conditions, what happens to the pyruvate produced from glycolysis? |
Enters TCA cycle and the 2 NADH produces goes to the ETC |
|
|
|
How can glycolysis be split into 2 phases? |
1. An energy investment phase 2. An energy generation phase |
|
|
|
In anaerobic conditions, how is the pyruvate converted to lactate? |
By lactose dehydrogenase and NADH |
|
|
|
Write down the pathway of glycolysis (energy investment stage? |
Glucose -> G-6-p -> F-6-p -> F-1,6-bisp -> glyceraldehyde-3-p + dihydroxyacetone phosphate Uses 2ATP at 1 and 3 |
|
|
|
What are the enzymes at each stage? |
Hexokinase, phosphohexose isomerise, phospho-fructokinase-1, alsolase, triose phosphate isomerase |
|
|
|
Which glyceraldehyde-3-p + dihydroxyacetone phosphate is converted into the other for progression into the second part of glycolysis? |
Dihydroxyacetone phosphate is converted into glyceraldehyde-3-p |
|
|
|
What are the main storage sites of glucose? |
Liver Skeletal muscle |
|
|
|
How does glucose enter cells? |
Facilitated glucose transporter GLUT |
|
|
|
What initially traps the glucose inside the cell? |
Conversion immediately by hexokinase |
|
|
|
Name the different GLUT transporters for different organs? |
GLUT1 - liver, beta cells, hypothalamus GLUT2 - neurones, placenta, testes GLUT4 - skeletal and cardiac muscle, fat GLUT5 - mucosal surface of small intestines |
|
|
|
If glucose stores are replete, what happens to the extra glucose? |
Stored as fat |
|
|
|
What is the daily consumption of glucose at rest? |
160g |
|
|
|
How much glucose does the brain use per day? |
70% (120g) |
|
|
|
How does skeletal muscle get glucose for energy? |
It’s own stores Glucose generated in the liver via the Cori cycle |
|
|
|
Can the glucose stored in the skeletal muscle be released into the blood? |
NO For own use only |
|
|
|
Do red blood cells use glucose for energy? |
Yes |
|
|
|
Why do red blood cells need glucose? |
Have no mitochondria and can only make ATP by glycolysis |
|
|
|
What do the red blood cells convert glucose to? |
Lactate |
|
|
|
Give an overview of the second phase of glycolysis |
Glyceraldehyde-3-phosphate is converted into pyruvate Produces 2NADH, 2ATP, 2ATP (substrate level phosphorylation) |
|
|
|
Why is aerobic glucose oxidation important in the brain? |
High energy requirements Cannot has FA |
|
|
|
What is the pentose phosphate pathway? |
Generates NADPH from glucose-6-p needed by lipogenesis Also generates ribose-5-poor synthesis of nucleotides Part of glycolysis |
|
|
|
Why is the generation of NADPH from the pentose phosphate pathway important? |
Recycling of the antioxidant glutathione |
|
|
|
What are the bonds at the branches of glycogen that are broken? |
Alpha 1-4-glycosidic bonds |
|
|
|
Where is glycogen synthesised? |
Liver and muscle |
|
|
|
What are the other fates of pyruvate other than acetyl-CoA? |
Conversion to lactate (anaerobic conditions) Conversion to oxaloacetate (replenish TCA intermediates) Conversion to alanine (by trans animation) |
|
|
|
How is glycogen broken down? |
Liberation of G1P molecule catalysed by glycogen phosphorylase |
|
|
|
What enzymes is used to breakdown glycogen? |
Glycogen phosphorylase and transferase |
|
|
|
When pyruvate is converted into acetyl-CoA, what are the byproducts? |
1NADH and 1CO2 per pyruvate! |
|
|
|
When is glycogen phosphorylase or transferase used to break down glycogen? |
Glycogen phosphorylase- remove glucose residues from free chain ends until it is four residues from a branching point Then transferase moves three residues to an adjacent chain for future breakdown by glycogen phosphorylase |
|
|
|
How is glycogen synthesis controlled in the liver and muscle? |
Intrinsic regulatioextrjnsic regulation |
|
|
|
What is the pentose phosphate pathway? |
Generates NADPH from glucose-6-p needed by lipogenesis Also generates ribose-5-phosphate synthesis of nucleotides Parallel to glycolysis, important in RBC |
|
|
|
What does glycolysis yield? |
2ATP, 2NADH, 2 pyruvate |
|
|
|
Under aerobic conditions how many ATP molecules are generated from a molecule of glucose? |
36ATP |
|
|
|
What enzyme commits pyruvate to acetyl-CoA |
Pyruvate dehydrogenase |
|
|
|
What happens to the converted Acetyl-CoA? |
Put into TCA cycle |
|
|
|
What are the other dates of pyruvate other than acetyl-CoA? |
Conversion to lactate (anaerobic conditions) Conversion to oxaloacetate (replenish TCA intermediates) Conversion to alanine (by trans animation) |
|
|
|
How is the enzyme pyruvate dehydrogenase inactivated? |
By phosphorylation |
|
|
|
What can phosphorylate pyruvate dehydrogenase? |
Kinase activates by ATP, acetylCoA and NADH |
|
|
|
How is glycogen synthesis controlled in the liver and muscle? |
Intrinsic regulation Extrinsic regulation |
|
|
|
What is involved in the intrinsic regulation of glycogen synthesis? |
Allows cells to respond to their own energy needs by breaking down glycogen when cell ATP and glucose levels fall and switches on glycogen synthesis when the concentrations rise |
|
|
|
What is the extrinsic regulation pathway of glycogen synthesis |
Mediates by hormones or other stimuli Increases in intracellular Ca2+ or cAMP will promote glycogen breakdown and inhibit synthesis Insulin signals the fed state and enhances glycogen synthesis and inhibits breakdown this storing energy for the future |
|
|
|
What controls glycogenolysis? |
Glycogen phosphorylase can exist in an activated or inactivated form via PKA or Ca2+ |
|
|
|
What controls glycogenesis? |
Glycogen synthase can exist in 2 forms, activated and inactivated Via PKA, phosphorylase kinase etc |
|
|
|
What glucose related process does adrenaline and glucagon promote? |
Glycogenolysis |
|
|
|
What glucose related process does insulin promote? |
Glycogen synthesis |
|
|
|
What is gluconeogenesis? |
Synthesis of glucose from non-carbohydrate precursor |
|
|
|
Provide more information on the glucose-fatty acid cycle in adipose tissue |
When plasma glucose concentration is high, the plasma insulin concentrations responds 1. Insulin suppresses fat metabolism (by the release of FA from adipose) 2. Therefore a high plasma glucose concentration leads to a low plasma FA concentration |
|
|
|
Provide more information on the glucose-fatty acid cycle in muscle |
Rate at which FAs are utilised from plasma is dependent almost entirely on the plasma FA concentration Thus addition of glucose becomes available in the plasma (e.g. after a meal, muscle will tend to switch to the use of glucose rather than fatty acids because firstly glucose uptake will be stimulated by insulin and secondly plasma NEFA concentrations will fall and remove that substrate) |
|
|
|
What happens between meals for the glucose-fatty acid cycle? |
Plasma glucose falls a little. Insulin secretion decreases and the plasma NEFA concentrations rise. Sore carbs for tissues such as brain that cannot use FA |
|
|
|
Draw the glucose-fatty acid cycle |
Draw |
|
|
|
Why is gluconeogenesis important in the liver and kidney? |
Liver - synthesises glucose for export to other glucose-dependent tissues during starvation and intense exercise Renal cortex - also produces about 10% of glucose from gluconeogenesis |
|
|
|
Which tissues are prominent in |
Adipose tissue is prominent Skeletal muscle is lacking |
|
|
|
Is gluconeogenesis the opposite of glycolysis? |
No! Has bypass reactions |
|
|
|
What is ne novo lipolysis? |
Synthesis of FAs and TAGs from substrates other than lipids |
|
|
|
From one molecule of glucose, how many acetyl-CoA’s are made? |
2 |
|
|
|
What tissue is FA oxidation prominent and which are lacking? |
Skeletal muscle have a high capacity Adipose has none |
|
|
|
W |
Adipose tissue is prominent Skeletal muscle is lacking |
|
|
|
What does an increased concentration of malonyl-CoA do? |
Divert those FA reaching the liver into esterification rather than oxidation (via inhibition of CPT-1) |
|
|
|
What is the glucose-fatty acid cycle? |
Metabolic interactions between glucose and fat metabolism |
|
|
|
Draw the glucose-fatty acid cycle |

Draw |
|
|
|
How much protein is consumed in the diet daily? |
70g |
|
|
|
How are dipeptides and tripeprides taken up in the intestines? |
Protein-coupled co-transporter on the enterocytes |
|
|
|
Once the dipeptides and tripeptides cross the enterocytes, what happens to them to be put into the circulation? |
Cleaves by intracellular peptidases into free amino acids. The free amino acids leave via the basolateral membrane and enter the circulation |
|
|
|
How are free amino acids taken up in the intestines? |
Absorbed by a number of mainly sodium-coupled transport systems into the enterocyte |
|
|
|
What does essential amino acids mean? |
The body cannot make |
|
|
|
What are the non-essential amino acids? (10) |
Arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine |
|
|
|
What happens if there is a lack of essential amino acids? |
Inability to synthesis proteins containing that amino acid |
|
|
|
What are non-essential amino acids? |
Can be made by the body |
|
|
|
What are the non-essential amino acids? (10) |
Alanine, aspartate, asparagine, cysteine, glutamate, glycine, proline, serine, tyrosine |
|
|
|
What are some example of what amino acids can be incorporated into? |
Protein synthesis Hormones e.g. adrenaline Neurotransmitters |
|
|
|
How can amino acids be deaminated and the carbon skeleton by? |
Oxidation via the TCA cycle Converted into glucose via gluconeogenesis Turned into fatty acids |
|
|
|
What is the enzyme secreted by the stomach to break down protein? |
Pepsin is secreted in the stomach in an inactive form (pepsinogen) which is activated by cleavage of a peptide fragment from its amino terminus |
|
|
|
How are amino acids stored as? |
Trick question They cannot be stored |
|
|
|
How can the carbon skeleton of amino acids be used as an energy source? |
Transamination |
|
|
|
What is the process of transamination? |
Amino group of amino acid carbon skeleton is removed and excreted as urea Each amino acid has its own specific aminotransferase |
|
|
|
Can |
Alpha-ketoglutarate, which forms glutamate |
|
|
|
What happens to the carbon skeleton after the amino group has been removed? |
They get converted into glucose or fatty acids for oxidation or storage |
|
|
|
Can all amino acids fit int pall stages of the TCA cycle to be made into FA/glucose? |
No |
|
|
|
What are the amino acids (carbon skeleton) called when they can be degraded to pyruvate or TCA cycle intermediates? |
Glucogenic |
|
|
|
What are the amino acids (carbon skeleton) called when they can be converted into acetyl-CoA or acetoacetate-CoA? |
Ketogenic |
|
|
|
What amino acids are gluconeogenic? (Via pyruvate) |
Tryptophan, alanine, cysteine, glycine, serine, threonine |
|
|
|
What amino acids are gluconeogenic? (Via TCA cycle intermediates) |
Oxaloacetate: asparagine, aspartate Fumerate: aspartate, phenylalanine, tyrosine Succinate: isoleucine, methionine, threonine, valine Alpha-ketoglutarate: arginine, glutamate, glutamine, histidine, proline |
|
|
|
Now is pepsin activates? |
Auto activated with pH<5 or by active pepsin |
|
|
|
What amino acids are ketogenic? |
Only leucine and lysine strictly Acetyl-CoA: isoleucine, leucine, tryptophan Acetoacetate: leucine, lysine, phenylalanine, tyrosine |
|
|
|
Which amino acids are mixed? |
Isoleucine, tryptophan, phenylalanine, tyrosine |
|
|
|
Why is glutamate an important acceptor of the amino group of amino acids during energy production? |
Provides a pool of amino groups for making other non-essential amino acids Also for deamination |
|
|
|
How is glutamate deaminated by? |
Glutamate dehydrogenase |
|
|
|
What does the deamination of glutamate generate? |
Alpha-ketoglutarate and NH4+, NADH |
|
|
|
What regulates glutamate dehydrogenase? |
Allosterically bu increases in ADP and GDP |
|
|
|
What happens to the ammonia produced by deamination of glutamate? |
Incorporated into urea for excretion |
|
|
|
Where does deamination of glutamate take place? |
Mitochondria of liver cells |
|
|
|
How is nitrogen excreted? |
Via idea of ammonium ions |
|
|
|
Why are other sites of ammonium production? |
Brain - breakdown of GABA neurotransmitter (ammonia used to form glutamate and transported to the liver) Muscle - ammonia formed from natural protein turnover, muscle catabolism during starvation and from the breakdown of excess ADP during severe exercise Intestinal cells - glutamate serves as an energy source |
|
|
|
What secretes endopeptidases? |
Pancreas |
|
|
|
How does the renal cortex deaminated glutamate? |
The ammonium is used to assist with acidifying the urine Conserves HCO3- |
|
|
|
Where is urea generated? |
Liver |
|
|
|
Why is excretion of excess proteins important? |
Cannot store it Can be toxic |
|
|
|
What should blood levels of free ammonia be? |
25-40uM |
|
|
|
What happens if levels of ammonium ion rise? |
NH4+ reacts with alpha-ketoglutarate to form glutamate. At these high levels, The brain reduces the rate at which ATP can be formed, therefore cells are starved of energy |
|
|
|
Approximately how much of the excess nitrogen is excreted as urea? (%) |
80% |
|
|
|
What is the urea cycle? |
Describes the formation of urea from one free ammonium ion and one donated from aspartate |
|
|
|
What is the first reaction of the urea cycle? |
Carbonyl group from carbamoyl phosphate is transferee to ornithine to form citrulline Catalysed by ornithine transcarbamoylase |
|
|
|
What is the second reaction of the urea cycle? |
Activates cotrulline by forming a citrullyl-AMP intermediate, the intermediate is attacked by the amino group of an aspartate residue to form argininosuccinate Catalysed by argininosuccinate synthesise (also uses ATP) |
|
|
|
What is the third step of the urea cycle? |
Cleaves argininosuccinate into fumarate and arginine Catalysed by argininosuccinate |
|
|
|
What are the endopeptidases secreted by the pancreas? |
Trypsin, chymotrypsin, elastase |
|
|
|
What is the final step of the urea cycle? |
Cleaves arginine to produce urea and ornithine Catalysed by arginase |
|
|
|
Draw the overall urea cycle |
Draw |
|
|
|
Wh |
Carboxypeptidases A and B |
|
|
|
What do endopeptidases do to protein? |
Cleave internal peptide bonds and release large peptide fragments |
|
|
|
What do exopeptidases do? |
Break short chains down from the end |
|
|
|
H |
Of 10kg of protein in the body. There is a continuous synthesis and breakdown of 300g per day (I.e. 3% turnover) |
|
|
|
Draw the overall urea cycle |
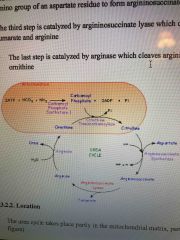
Draw |
|
|
|
Where does the urea cycle take place? |
Partly in the mitochondrial matrix and partly in the cytoplasm |
|
|
|
What are the purines? |
Adenine, guanine (2x phosphate rings) |
|
|
|
What are the pyrimidines? |
Cytosine, thymine (DNA only), uracil (RNA only) |
|
|
|
How are nucleic acids synthesised? |
Pentose phosphate pathway generates ribose-5-phosphate for the synthesis of nucleotide |
|
|
|
How are purines catabolised? |
Purines are degrades to urate via uric acid, catalysed by xanthine oxidase |
|
|
|
How are pyrimidines catabolised? |
Cytosine and uracil are converted into beta-alanine the. Malonyl-CoA (needed for FA synthesis) Thymine is conveyed into beta-aminisobutyric acid which is then Used to form methylmalonyl-CoA The leftover carbon skeletons are oxidised in TCA cycle Pyrimidines degradation ends in the formation of ammonium, water and carbon dioxide. The ammonium enters urea cycle |
|
|
|
What is the end point of purine degradation? |
Uric acid |
|
|
|
How is uric acid important in Gout? |
Elevated levels of plasma (urate) Joint inflammation due to deposition of sodium urate crystals Also renal disease due to deposition of these crystals here |
|
|
|
Why does Gout happen? |
Excess urate production - due to variety of inborn errors of metabolism Or due to deficiency in HGPRT (salvage enzyme) |
|
|
|
How do you reduce your purine intake? |
Avoid eating meat, fish, protein |
|
|
|
What fuel does the heart prefer? |
NEFAs and endogenous triglycerides |
|
|
|
What happens during later fasting state/ starvation? |
Liver is already gluconeogenic but also becomes ketogenic and peoteolytic Ketone bodies formed from FA released by lipolysis in adipose FA -> fuel in muscle Ketone bodies -> fuel in brain and muscle Glycerol from TAG breakdown is used by liver for gluconeogenesis Protein hydrolysis takes place in the muscle and alanine and glutamine are release Alanine -> alanine cycle Glutamine -> metabolised by enterocytes |
|
|
|
What is leptin? |
Peptide hormone secreted by adipocytes in response to the amount of fat stored. Increased fat strokes leads to increases in circulating leptin concentrations |
|
|
|
What does leptin do? |
Acts through hypothalamic receptors and a complex neuroendocrine system to reduce appetite |
|
|
|
What is leptin deficiency associated with? |
Intense hunger and obesity |
|
|
|
What regulates carbohydrate response to nutritional status? |
Pyruvate kinase, pyruvate dehydrogenase, FA synthesis, insulin |
|
|
|
What regulates insulin response elements to nutritional status? |
Hexokinase, acetyl-CoA carboxylase, G-6-P, PPARS |
|
|
|
What does thyroid hormone do? |
Stimulate diverse metabolic activities leading to an increase in basal metabolic rates |
|
|
|
What metabolic activities does thyroid hormone stimulate? |
Lipid metabolism ( increases fat metabolism, FA oxidation) Carbohydrates metabolism (increases gluconeogenesis and glycogenolysis) |
|
|
|
What is the thyroid hormone important in? |
Growth in children Development of fetal brain Cardiovascular system (HR, CO) Reproductive system (infertility) |
|
|
|
What can cause hypothyroidism? |
Iodine deficiency Primary thyroid disease |
|
|
|
What happens to the energy requirements of skeletal muscle during anaerobic exercise? |
Energy for muscle contraction comes from intramuscular stores (glycogen and phosphocreatine) Due to lack of oxygen. Muscle cells are glycolytic, producing a lot of lactic acids which cause cramps and weakening. This drops intracellular pH which inhibits glycolysis. And limits lactate formation |
|
|
|
What are the symptoms of hypothyroidism? |
Goiter Lethargy, fatigue, weakness, hair loss, reproductive failure |
|
|
|
What is the most common form of hyperthyroidism? |
Graves’ disease |
|
|
|
What is Graves’ disease? |
Autoimmune- autoantibodies Bind and activate thyroid stimulating hormone receptor, leading to continuous thyroid hormone synthesis |
|
|
|
What are the symptoms of hyperthyroidism? |
Nervousness, insomnia, high HR, eye disease, anxiety |
|
|
|
What is cortisol? |
Steroid hormone produced by zona fasciculata of the adrenal cortex within the adrenal gland Released in response to stress and low blood-glucose concentration |
|
|
|
What are catecholamines? |
Adrenaline, noradrenaline, dopamine |
|
|
|
What is T1DM |
Caused by defective or absent pancreatic beta-cells |
|
|
|
What are the symptoms of T1DM? |
Hyperglycaemia Hyperlipoproteinaemia Severe ketoacidotic episodes |
|
|
|
What happens to the energy requirements of skeletal muscle during aerobic exercise (short to moderate term)? |
Glucose from muscle glycogen is the main source of energy Glucose uptake from the blood is also increased by non-insulin-dependent insertion of GLUT4 into the muscle cell plasma membrane Glucose is released from liver glycogenolysis Muscle also increased oxidation of branched-chain amino acids. Ammonium production and alanine release |
|
|
|
What happens to the energy requirements of skeletal muscle during aerobic exercise (long term)? |
As there is not enough stored glycogen in skeletal muscle form long term exercise... Increase in lipolysis - FA used correctly as fuel by muscle or as ketone bodies Progressive switch over to preferential fatty acid oxidation by muscle |
|
|
|
During feeding, what happens to glucose, amino acids and lipids? |
Glucose and amino acids are taken up by the liver by the portal vein before entering general circulation. A rise in blood glucose triggers insulin release Lipids are absorbed via the lymph system which drains into the vena cava, transported in lymph by chylomicrons |
|
|
|
During feeding, what happens to glucose? |
Taken up by liver and stored as glycogen Can be metabolised to pyruvate en route to fat synthesis TAGs synthesised in the liver are carried in the blood in the form VLDLs Glucose continues into the circulation Used by the brain and testes (solely glucose users) and RBC, renal medulla Insulin triggers insertion of GLUT4 transporters to increase uptake capacity Glucose is also taken up by adipocytes for conversion into fat |
|
|
|
During feeding, what happens to protein? |
Taken up by liver Used by liver for protein synthesis Deamination to give alpha-ketoacid (ultimately pyruvate) and ammonia Pyruvate used for fat and glucose/glycogen synthesis; ammonia excreted via urea cycle Protein is then passes into main circulation for protein synthesis in all tissues |
|
|
|
During feeding, what happens to lipids? |
TAGs are absorbed, via the lymph and enter the main circulation Taken up by adipose tissue and stored Used by muscle as an energy source |
|
|
|
What happens during fasting? |
Pancreatic alpha cells release glucagon, triggered by a fall in blood glucose (skeletal muscle does not react to glucagon) The liver no longer uses glucose as a fuel source Muscle switches to FA and ketone bodies, the brain starts to use ketone bodies RBC, Rena medulla are the only once’s using glucose still Protein is used from muscle as a last resort during starvation |
|
|
|
What happens during the early fasting state? |
During initial unfed state of fasting, liver glycogen is broken down and glucose is released into circulation Glucose is used by tissues (brain, RBC, muscle) The alanine cycle becomes important (alanine generates fromcamination is pyruvate) the alanine is taken up by the liver and deaminated, nitrogen excreted as urea and the pyruvate is conveyed to glucose by gluconeogenesis The Cori cycle operates |
|

