![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
101 Cards in this Set
- Front
- Back
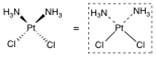
What structure is this?
|
Cisplatin
(Square-planar geometry |
|
|
Organoplatinum antineoplastic agents contain what and act as what?
|
an electron-deficient platinum atom that acts as a magnet for electron-rich DNA nucleophiles.
|
|
|
The platinum atom in cisplatin is associated with what two functional group ligands?
|
with two chlorine ligands and two ammonia ligands
|
|
|
Platinum is inherently electron deficient, but the net charge on the organometallic complex is ____, why?
|
zero because of the contribution of electrons by two of the four ligands (the chlorines) bound to platinum.
|
|
|
The ammonia-platinum bonds in cisplatin are what kind of bonds?
|
dipolar bonds (aka dative covalent bond, coordinate bond) is a kind of 2-center, 2-electron covalent bond in which the two electrons derive from the same atom.
|
|
|
Like nitrogen mustards, organoplatinum complexes are ____ and can accept electrons from two ___ ___
|
are bifunctional and can accept electrons from two DNA nucleophiles.
|
|
|
All of the currently marketed organoplatinum anticancer agents are Pt(II) complexes with what kind of geometry?
|
square-planar
|
|
|
Cisplatin Bioactivation and Intrastrand DNA Cross-Linking
The chloride ions in cisplatin are particularly important in its action as a drug. They are relatively stable when the drug is what? |
is outside the cell, where the chloride concentration
is normally high |
|
|
The chloride ions in cisplatin are particularly important in its action as a drug. They are relatively stable when the drug is outside the cell, where the chloride concentration
is normally high. But when the drug gets inside the cell and the chloride concentration drop, what happens to the cisplatin? |
cisplatin loses its two chlorides, and they are replaced by water molecules
|
|
|
The chloride ions in cisplatin are particularly important in its action as a drug. They are relatively stable when the drug is outside the cell, where the chloride concentration
is normally high. But when the drug gets inside the cell and the chloride concentration drops, cisplatin loses its two chlorides, and they are replaced by water molecules. What does this result in formation of? |
mono- and di-hydrated cisplatins are the cytotoxic agents which give cisplatin its anticancer effect.
|
|
|
Cisplatin Bioactivation and DNA Cross-Linking
Because the original chlorine anions leave the metal, the hydrated organoplatinum molecule has what kind of charge? What readily happens to it? |
has a net positive charge and is readily attacked by DNA nucleophiles (e.g., the N7 of adjacent guanine residues
|
|
|
Because the original chlorine anions leave the metal, the hydrated organoplatinum molecule has a net positive charge and is readily attacked by DNA nucleophiles (e.g., the N7 of adjacent guanine residues). What is the net result
|
is a major change in DNA conformation such that base pairs that normally engage in hydrogen-bond formation are not permitted to interact.
|
|
|
Because the original chlorine anions leave the metal, the hydrated organoplatinum molecule has a net positive charge and is readily attacked by DNA nucleophiles (e.g., the N7 of adjacent guanine residues). The net result is a major change in DNA conformation such that base pairs that normally engage in hydrogen-bond formation are not permitted to interact.
What how are the 2 amine ligands of the complex bound? What do they stabilize? By doing what? |
but they do stabilize the cross-linked DNA-platinum complex by forming strong ion–dipole bonds with the anionic (negatively charged) phosphate residues on DNA.
|
|
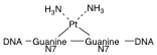
What is this structure?
|
cisplatin geometry predisposed to crosslink DNA
|
|
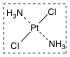
What is this structure?
|
transplatin
|
|
|
Because of the cis geometry of the chlorine-platinum bonds, cisplatin easily forms what?
|
forms crosslinks
between DNA bases. |
|
|
Transplatin has a trans arrangement of chlorine atoms and that is the reason why transplatin is an ineffective what?
|
an ineffective anticancer chemotherapeutic.
|
|
|
Intrastrand cross-links most frequently occur between what?
|
adjacent guanine residues referred to as diguanosinedinucleotides (60–65%) or adjacent guanine and adenine residues (25–30%).
|
|
|
How often does intErstrand cross-linking occur compared to intrAstrand? What bases does intrEstrans involve?
|
which occurs much less frequently (1–3%),usually involves guanine and adenine bases.
|
|
|
Many of the antineoplastic antibiotic compounds inhibit what?
|
topoisomerase II
|
|
|
What is topoisomerase II?
|
an enzyme responsible for maintaining proper DNA structure during replication and transcription to RNA.
|
|
|
Topoisomerase II normally cleaves DNA during what phase?
|
replication
|
|
|
Topoisomerase II normally cleaves DNA during the replication phase but repairs its own what?
|
damage after replication is complete
|
|
|
Topoisomerase II inhibitors act to stimulate what? What do they inhibit? What does this leave?
|
the cleavage reaction but inhibit the DNA resealing activity of the enzyme
leaving the DNA irreversibly damaged and unable to replicate. |
|
|
Yet another proposed mechanism of cytotoxic action is the generation of what? What does this cause?
|
cytotoxic free radicals that cause single-strand breaks in DNA.
|
|
|
One antibiotic _______ is capable of alkylating DNA
|
mitomycin
|
|
|
To bulldoze its way between the bonded DNA strands and cause a chemical change in DNA structure, a segment of the antibiotic must have what?
|
the trigonal coplanar geometry guaranteed by aromaticity.
|
|
|
Antibiotic antineoplastics that interact directly with DNA first intercalate what?
|
the double-stranded helix
|
|
|
Antibiotic antineoplastics that interact directly with DNA first intercalate the double-stranded helix by inserting between what?
|
between the base pairs and forming strong noncovalent interactions with DNA bases
|
|
|
Antibiotic antineoplastics that interact directly with DNA first intercalate the double-stranded helix by inserting between the base pairs and forming strong noncovalent interactions with DNA bases. The highly stabilized complex deforms what? What does this prohibit?
|
deforms the DNA, prohibiting proper replication.
|
|
|
Anthracycline antineoplastic antibiotics are very closely related to what?
|
to the tetracycline antibacterials
|
|
|
Anthracycline antineoplastic antibiotics are very closely related to the tetracycline antibacterials. Structurally, they are ____ and contain a ____ and a ____organic portion.
|
glycosides
sugar portion nonsugar |
|
|
Anthracycline antineoplastic antibiotics are very closely related to the tetracycline antibacterials. Structurally, they are glycosides and contain a sugar portion (L-daunosamine) and a nonsugar organic portion. The nonsugar portion of glycosides is generically referred to as an _____?
|
aglycone
|
|
|
In anthracyclines, the aglycone moiety is specifically called ____
|
anthracyclinone or anthroquinone.
|
|
|
Intercalation is most common in DNA regions that are rich in what?
|
guanine and cytosine bases
|
|
|
Antobiotics – Anthracyclines and Anthracenediones
The predominant cytotoxic effect is not due to the intercalation of this molecule in DNA, but the inhibition of _____? |
topoisomerase II
|
|
|
. Anthracyclines bind to the DNA-enzyme complex in the area close to the DNA cleavage site. They stabilize what complex? What does this allow to happen to the DNA? What does it link to? What does it not permit?
|
a ternary cleavable complex that allows the DNA to be cut and covalently linked to topoisomerase Tyr residues but that does not permit the cleaved DNA to reseal.
|
|
|
The aromatic portion of the anthracyclinone ring system and the daunosamine sugar bind to _____
Where as anthracyclinone A ring is believed to bind with the ____ enzyme? |
DNA
topoisomerase II |
|
|
A very important mechanism of use-limiting anthracycline cardiotoxicity involves the formation of what?
|
cytotoxic free radicals
|
|
|
A very important mechanism of use-limiting anthracycline cardiotoxicity involves the formation of cytotoxic free radicals. NADPH/P450 reductase is the ____ for ____ enzymes
|
electron donor protein for several oxygenaseenzymes
|
|
|
When NADPH/CYP450 reductase reduces the quinone ring to a hydroquinone,___ ___ ___ are generated.
|
superoxide radical anions
|
|
|
Superoxide radical anions react to generate ___ ___? What does this reaction require? What is it catalyzed by?
|
hydrogen peroxide, a reaction that requires protons and is catalyzed by the enzyme superoxide dismutase in a Cu2+mediated process.
|
|
|
Anthracycline cardiotoxicity
In the presence of the enzyme catalase what happens to hydrogen peroxide? |
hydrogen peroxide is rapidly converted to water and oxygen.
|
|
|
In the presence of ferrious Ion (Fe2+) what happens to hydrogen peroxide? What is this reaction known as?
|
hydrogen peroxide is converted into the highly toxic hydroxyl radical through a process called the Fenton reaction.
|
|
|
Hydroxyl radicals promote what in DNA? Why is this desireable?
|
Hydroxyl radicals promote single-strand breaks in DNA, which is therapeutically desirable to treat the uncontrolled growth of cancer cells.
|
|
|
Anthracyclineanti-cancer agents also are known to interfere with what?
|
with normal ferritin-iron mobilization, resulting in iron accumulation
|
|
|
The anthracyclines chelate strongly with what ions? What reduction is essentially guaranteed
|
with di- and trivalent cations, including intracellularFe2+, so the generation of cytotoxic hydroxyl radicals after the initial NADPH/CYP450 reductase reduction is essentially guaranteed.
|
|
|
____ ____ and ___ ___also are formed during the production of hydroxyl radicals
|
Hydroxide anion and ferric ion (Fe3+)
|
|
|
___ ___ is particularly vulnerable to free radical damage by the anthracyclines
|
Cardiac tissue
|
|
|
Cardiac tissue is particularly vulnerable to free radical damage by the anthracyclines because why?
|
it does not contain the catalase enzyme.
|
|
|
Cardiac tissue is particularly vulnerable to free radical damage by the anthracyclines because it does not contain the catalase enzyme. When hydrogen peroxide forms in the myocardium, which pathway is it forced to go down?
|
Fenton Pathway
|
|
|
Cardiac toxicity is the major use-limiting side effect of what?
|
anthracycline use
|
|
|
Cardiac toxicity is the major use-limiting side effect of anthracycline use, but coadministration of ______ has been shown to lower its incidence.
|
dexrazoxane(anantioxidant and iron chelator)
|
|
|
Additionally, the active form of anthracycline is a secondary alcohol which acts as an active what?
|
cardiotoxic metabolite by disrupting the flow of myocardial cations.
|
|
|
The active secondary alcohol metabolite formed by aldoketo reductase affects what? By increasing what? and inhibiting what?
|
aldoketoreductase affects muscle tissue by increasing cytosolic levels of Ca2+ ions, and inhibiting Na,K–adenosine triphosphatase.
|
|
|
The active secondary alcohol metabolite formed by aldoketoreductase affects muscle tissue by increasing cytosolic levels of Ca2+ ions, and inhibiting Na,K–adenosine triphosphatase. Collectively, these cellular events can induce a chronic ____?
|
cardiomyopathy
|
|
|
Chemically, mitoxantrone is classified as an ____?
|
anthracenedione
|
|
|
mitoxantrone has the structural features needed to do what?
|
to intercalate DNA and inhibit topoisomerase II,
|
|
|
This molecule has the structural features needed to intercalate DNA and inhibit topoisomerase II, but the enhanced stability of the quinone ring (possibly through an increased potential for intramolecular hydrogen bonding) makes the ring highly resistant to what?
|
NADPH/CYP450 reductase.
|
|
|
This molecule has the structural features needed to intercalate DNA and inhibit topoisomerase II, but the enhanced stability of the quinone ring (possibly through an increased potential for intramolecular hydrogen bonding) makes the ring highly resistant to NADPH/CYP450 reductase. This limits the formation of what?
|
of the superoxide radicals that are required for the generation of the highly toxic hydroxyl radical.
|
|
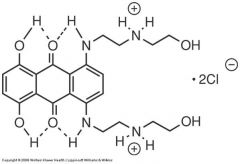
What structure is this?
|
Mitoxantrone
|
|
|
Mitomycin is used for what?
|
Antibiotic for cancer chemotherapy
|
|
|
Mitomycin is activated through what process?
|
bioreductive process
|
|
|
Mitomycin is activated through a bioreductive process utilizing what?
|
NADPH/CYP450 reductase and/or NAD(P)H quinoneoxidoreductase 1 (NQ01 reductase), an enzyme expressed in many neoplastic cells.
|
|
|
Mitomycin is activated through a bioreductive process utilizing NADPH/CYP450 reductase and/or NAD(P)H quinoneoxidoreductase 1 (NQ01 reductase), an enzyme expressed in many neoplastic cells. Through these enzymes, the quinone ring of mitomycin is reduced to what? What does this generate?
|
to the hydroquinone, generating superoxide radicals in the process that ultimately will be converted to cytotoxic hydroxyl radicals through the Fenton reaction.
|
|
|
Formation of the hydroquinone is followed by aromatization to the ___ ring, through the loss of ___?
|
indole ring through the loss of methanol.
|
|
|
Both the electrophilicaziridine ring and the methylene group adjacent to the carbamate ester are vulnerable to what? Such as what? What does this result in?
|
to attack by DNA nucleophiles, such as the 2-NH2 group of guanine or the 4-NH2 group of cytosine, resulting in cross-linked DNA and cell death.
|
|
|
What most commonly stop the de novo synthesis of DNA by inhibiting the formation of the nucleotides?
|
antimetabolites
|
|
|
The rate-limiting enzymes of nucleotide biosynthesis often are the primary target for what?
|
antimetabolites
|
|
|
Antimetabolites also are capable of inhibiting other enzymes required in what?
|
the biosynthesis of DNA
|
|
|
Antimetabolites also are capable of inhibiting other enzymes required in the biosynthesis of DNA and many can arrest __ ___ by promoting the incorporation of what?
|
chain elongation by promoting the incorporation of false nucleotides into the growing DNA strand.
|
|
|
The antimetabolites serve as false substrates for what enzymes?
|
critical nucleotide biosynthesis enzymes
|
|
|
Purine antagonists inhibit what?
|
the synthesis of the purine-based nucleotides adenylatemonophosphate (AMP) and guanylatemonophosphate (GMP
|
|
|
Pyrimidine antagonists stop the production of what?
|
of the pyrimidine-based nucleotides, primarily deoxythymidinemonophosphate (dTMP)
|
|
|
dTMP synthesis inhibitors inhibit what? What does this result in?
|
inhibit thymidylate synthase either directly or indirectly, and this will result in a“thymineless death” in actively dividing cells.
|
|
|
Without dTMP and its deoxythmidinetriphosphate metabolite what happens to the DNA?
|
DNA will fragment, and the cell will die.
|
|
|
Deoxythymdinemonophosphate (dTMP) is biosynthesized via what?
|
C5-methylation of deoxyuridinemonophosphate (dUMP)
|
|
|
The rate-limiting enzyme of the dTMP synthetic pathway is what?
|
the sulfhydryl-containing thymidylatesynthase, with 5,10-methylene tetrahydrofolate(5,10-methylene-THF) serving as the methyl-donating cofactor.
|
|
|
The breakdown of the ternary complex (enzyme-substrate-cofactor) yields ___ and ___
|
a dTMP precursor and 7,8-dihydrofolate (DHF).
|
|
|
The dTMP precursor rearranges to ____? What does this liberate?
|
dtmp
liberating active thymidylate synthase enzyme, and the 7,8-DHF is converted back to the 5,10-methylene-THF cofactor |
|
|
active thymidylate synthase enzyme, and the 7,8-DHF is converted back to the 5,10-methylene-THF cofactor
|
dihydrofolatereductase (DHFR) enzyme.
|
|
|
Pyrimidine Antagonists - Direct Inhibitors of Thymidylate Synthase
Fluorouracil To bind to thymidylate synthase, fluorouracil prodrug must be converted to what form? |
deoxyribonucleotide form
|
|
|
The active form of fluorouracil differs from the endogenous substrate only by the presence of what? What is this the key too?
|
the 5-fluoro group, which holds the key to the cell-killing action of this drug
|
|
|
. The C6 position of the false substrate is significantly more ____ because why?
|
electrophilic than normal because of the strong electron-withdrawing effect of the C5 fluorine.
|
|
|
The C6 position of the false substrate is significantly more electrophilic than normal because of the strong electron-withdrawing effect of the C5 fluorine. This greatly increases the rate of attack by what?
|
the cysteine of thymidylate synthase
|
|
|
. The C6 position of the false substrate is significantly more electrophilic than normal because of the strong electron-withdrawing effect of the C5 fluorine. This greatly increases the rate of attack by the cysteine of thymidylatesynthase, resulting in a very fast formation of what?
|
a fluorinated ternary complex
|
|
|
Pyrimidine Antagonists - Direct Inhibitors of ThymidylateSynthase
Fluorouracil The next step in the dTMP synthesis pathway requires the abstraction of what? |
C5proton by N10 of the cofactor, but this is no longer possible
|
|
|
The next step in the dTMP synthesis pathway requires the abstraction of the C5proton by N10 of the cofactor, but this is no longer possible. Not only is the C5 fluorine bond stable to cleavage, the fluorine atom and N10 would repel one another because why?
|
they are both electron rich
|
|
|
The next step in the dTMP synthesis pathway requires the abstraction of the C5proton by N10 of the cofactor, but this is no longer possible. Not only is the C5 fluorine bond stable to cleavage, the fluorine atom and N10 would repel one another because they are both electron rich. The false ternary complex cannot do what? Is something formed? What is released? What is not regenerated?
|
break down, no product is formed, nocofactor is released, and most importantly, the rate-limiting enzyme (thymidylatesynthase) is not regenerated
|
|
|
Floxuridine prodrug is bioconverted via what? What is it converted to?
|
via 2-deoxyuridine kinase–mediated phosphorylation to the same active 5-fluoro-dUMP structure generated in the multistep biotransformation of fluorouracil
|
|
|
capecitabine is a carbamylated analogue of of what?
|
cytidine
|
|
|
Although capecitabine is a carbamylated analogue of cytidine, the drug actually is another ______ prodrug?
|
5-fluoro-dUMP
|
|
|
Although capecitabine is a carbamylated analogue of cytidine, the drug actually is another 5-fluoro-dUMP prodrug. Given orally, it is extensively metabolized to fluorouracil, which is then converted to what?
|
to the active fluorinated deoxyribonucleotide
|
|
|
Thymidine phosphorylase, an enzyme involved in this biotransformation, is much more active in ___ than ____? what does this improce?
|
Thymidine phosphorylase, an enzyme involved in this biotransformation, is much moreactive in
|
|
|
Levels of active drug in the tumor can be up to ____ than in surrounding tissue, leading to a lower incidence of side effects compared to fluorouracil therapy.
|
3.5-fold higher
|
|
|
What is methotrexate?
|
Methotrexate is a folic acid antagonist structurally designed to compete successfully with 7,8-DHF for the DHFR enzyme.
|
|
|
Methotrexate is a folic acid antagonist structurally designed to compete successfully with 7,8-DHF for the DHFR enzyme. The direct inhibition of DHFR causes what to build up? Which in turn results in what?
|
cellular levels of 7,8-DHF to buildup, which in turn results in feedback (indirect) inhibition of thymidylatesynthase.
|
|
|
The monoglutamate tail of methotrexate permits what?
|
active transport into cells
|
|
|
The monoglutamate tail of methotrexate permits active transport into cells. Once inside the cell, methotrexate undergoes what? What does this add?
|
a polyglutamation reaction that adds several anionic carboxylategroups to trap the drug at the site of action
|
|
|
The monoglutamate tail of methotrexate permits active transport into cells. Once inside the cell, methotrexateundergoes a polyglutamation reaction that adds several anionic carboxylategroups to trap the drug at the site of action. The C4 amino substituent of methotrexate enriches what?
|
electron density at N1 through electron donation
|
|
|
The monoglutamate tail of methotrexate permits active transport into cells. Once inside the cell, methotrexateundergoes a polyglutamation reaction that adds several anionic carboxylategroups to trap the drug at the site of action. The C4 amino substituent of methotrexate enriches electron density at N1 through electron donation, increasing its basic character between __ and ___ fold? What else does it promote?
|
10- and 1,000-fold and promoting protonation at N1 by the key amino acid residue (Asp27) of DHFR.
|

