![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
38 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What are structural formulas for molecules involving H, C, N, O, F, S, P, Si, Cl? |
Structural formulas show every atom and every bond, as well as the unshared electron pairs found in a molecule. Atoms are represented by their atomic symbol. Bonds are represented by solid black lines. A single black line or dash represents 2 shared electrons in a single covalent bond. Two black lines represent 4 shared electrons in a double covalent bond. Three black lines represent 6 shared electrons in a triple covalent bond. An unshared electron pair is represented by two dots paired together adjacent to the atomic symbol. |
|
|
|
Explain the concept of delocalized electrons and resonance in ions and molecules. |
Resonance structures result from electrons not being fixed in position (that's why you "push" electrons when drawing resonance structures).
When electrons are not fixed in position, they are delocalized electrons. For all practical purposes, resonance and electron delocalization mean the same thing. In ions, resonance and electron delocalization occurs to "distribute" the charge around. In molecules, resonance and electron delocalization occurs in aromatic rings and conjugated double bonds. Resonance of molecules distributes the electrons around to produce a hybrid structure. The electron and charge delocalization described by resonance enhances the stability of the molecules or ions. No atoms change their positions within the common structural framework. Only electrons are moved. The better resonance structure is that which has the least amount of formal charge. Just with electrostatics, like charges repel, so they like to be spread out as much as possible - this lowers the potential energy of the molecule. |
Huckel's rule: aromatic is planar monocyclic rings with 4n + 2 pi electrons (where n is any integer, including zero). |
|
|
What are isomers? |
Same molecular formula, different structural formula. |
|
|
|
What are constitutional isomers?
|
Constitutional isomerism in accordance with IUPAC, is a form of isomerism in which molecules with the same molecular formula have atoms bonded together in different orders, as opposed to stereoisomerism. Constitutional isomers are also called structural isomers, and they have the same molecular formula, but different connectivity.
Position isomers: structural isomers that have the same functional groups, but they are positioned differently. Functional group isomers: structural isomers that have the same molecular formula, but different functional groups. |
|
|
|
What are geometric isomers?
|
Geometric isomers have the same molecular formula, same connectivity, but have different orientation across a double bond. When both sides of the double bond contains the SAME 2 groups, then cis and trans is used. Cis = same side, Trans = opposite sides. When DIFFERENT groups are attached to either side of a double bone, Z and E is used.
Z is when the higher priority groups (ranked according to the Cahn-Ingold-Prelog rules) are orientated on the same side across the double bond. Zusammen is the German word for together. In the picture CH3 and Cl are the higher priority groups. Even if you have a double bond in the middle of the chain and it is difficult to see what side the groups are on, first look for the highest priority group period, like halogens, then compare that to the groups on the other side of the bond, which could be the carbon chain itself. E is when the higher priority groups are orientated on different sides across the double bond. Entgegen is the German word for opposed. For 1,2 substitution on a cyclohexane ring, axial and equitorial positions is a cis relationship. For a 1,2 substitution on a cyclohexane ring, axial and axial positions (opposite directions) as well as equitorial and equitorial positions (opposite directions) are trans configuraitons. The most stable configuration occurs when the largest groups are in the equitorial position. Even if it's a 1,2 substituted cyclohexane ring and the 2 groups are appear to have more steric hindrance in equitorial/equitorial than if it would if they were in axial/axial positions, equitorial positions are more stable..period! |
Geometric isomers have different physical properties. Cis molecules have a dipole moment while trans molecules do not. Due to their dipole moment, cis molecules have stronger intermolecular forces leading to higher boiling points (harder to boil to gas because of interactions). Due to their lower symmetry, however, cis molecules do NOT form crystals as readily, and thus have lower melting/freezing points (makes it harder to freeze/easier to melt, stays longer as a fluid). The substituent groups in the cis position may crowd each other; a condition known as steric hindrance. Steric hindrance in cis molecules produces higher energy levels resulting in higher heats of combustion.
|
|
|
What are meso compounds?
|
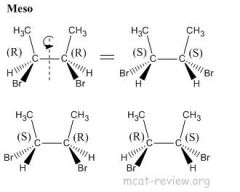
Meso compounds may have chiral centers, but as a molecule, they are achiral and optically inactive.
Meso compounds reduce the total number of stereoisomers. |
|
|
|
What are stereoisomers and chiral centers?
|
Stereoisomers have the same molecular formula, same connectivity, but have different 3-D arrangements across one or more asymmetric (chiral) centers. Stereoisomers are diastereomers, enantiomers, and cis/trans isomers (Geometric isomers). Chiral center is any atom with 4 different entities attached to it. You can't have stereoisomers if you don't have a chiral center. Enantiomers have the same chemical and physical properties (unless reactions with other chila compounds or reactions with polarized light). Geometric isomers have the same chemical properties, different physical properties. Diastereomers have different chemical and physical properties. The maximum number of optically active isomers that a single compound can have is equal to 2^(# of chiral centers). Two chiral centers in a single molecule may offset each other creating an optically inactive molecule. Such compounds are called meso compounds. Meso compounds have a plane of symmetry through their centers which divides them into two halves that are mirror images to each other. Meso compounds are achiral and therefore optically inactive.
|
|
|
|
What are enantiomers?
|
Enantiomers are mirror images of each other. That means ALL chiral centers in one enantiomer is reversed in the other. So if one enantiomer is R, the other is S. If two chiral centers if 1 enantiomer is R and S, the other is S and R.
Enantiomers have the same physical properties and chemical properties except for 2 cases: 1) reactions with ohter chiral compounds and 2) reactions with polarized light. |
|
|
|
What are diastereomers?
|
Diastereomers - more than one chiral center, inversion of stereochemistry on some but not all of its chiral centers. For examples, diastereomers would have stereochemistries of (R)-(R) vs (R)-(S). Another example of diastereomers would be (R)-(R)-(S)-(R) vs (R)-(R)-(R)-(R). Note: in biological molecules, people use D and L for R and S, respectively. Caution: D and L (absolute configurations) are NOT the same as d and l (relative configuration). The letters d and l are used for plane polarized light. Diastereomers have different physical properties and the same chemical properties. The absolute stereo configuration of the amino acids at the alpha carbon is typically referred to using the D/L notation with reference to the absolute configuration of Glyceraldehyde rather than the more modern R/S designation. Geometric isomers (cis/trans and E/Z) are diastereomers.
|
|
|
|
What are cis and trans isomers?
|
In rings, it is easier to assign stereoisomers as cis/trans rather than R or S. Cis is having the same groups on the same side of the ring. Trans is having the same groups on different sides of the ring. If there are two hydrogen groups on one end of the double bond, it cannot have geometric isomers.
Hydrogenation, when H2 adds to a double or triple bond, results in syn-addition, in which the 2 hydrogens add to the same face of the double bond - a cis molecule results. Or the result could be an alkane. The 2 hydrogens add to the same face of the double bond because hydrogenation must occur with a metal catalyst (Pt, Pd, Ni etc) and the 2 hydrogens are bound to the catalyst together when they approach the molecule. The metal catalyst increases the reaction rate by lowering the activation energy. High temperature aren't needed. The double bonds in benzene is even less reactive than regular alkenes because hydrogenation ruins the aromaticity of the molecule. Also, in order for benzene rings to maintain aromaticity, they undergo substitution reactions instead of addition reactions (a proton is substituted for something else). Also, if an alkene is reacted with an acid in a reaction, the alkene is the electrophile because it gets protonated by the acid and becomes a carbocation. |
|
|
|
What are conformational isomers?
|
Conformational isomers have the same molecular formula, same connectivity, same stereochemistry, but can rotate about a single bond to switch between different conformations.
Technically, conformational isomers are not really isomers because you don't have to break any bonds to convert from one conformation to another. They are more accurately called conformers. |
|
|
|
What are staggered and eclipsed conformations?
|
Conformers about a single bond |
|
|
|
What are chair, twisted boat, and boat conformations?
|
Conformers of cyclohexose
Chair: most stable, everything is staggered. Twist boat: less stable, things are not completely eclipsed. Boat: least stable, everything is eclipsed. Hexose rings will twist and turn to achieve the most stable conformation. |
|
|
|
What are axial and equatorial conformations?
|
Torsional strain: the strain due to eclipsing of groups across a single bond.
Steric interactions Axial: most unstable because the axial groups are orientated with a high degree of clashing. Equatorial: most stable because the equatorial groups are orientated away from one another. Bulky groups like to be in the equatorial position. Most stable conformation: completely staggered (chair), with bulky groups in the equatorial position. Least stable conformation: completely eclipsed (boat), with bulky groups in the axial position. |
|
|
|
What is specific rotation?
|
Specific rotation: chiral molecules containing a single enantiomer will rotate polarized light (to varying degrees) either to the left or to the right. This is why chiral molecules are said to be "optically active".
Left rotation: (-) or l or levorotatory. Right rotation: (+) or d or dextrorotatory. Caution: (+) or (-) does NOT correspond to R/S configurations. Caution: d and l is NOT the same as D and L. The upper case letters denote absolute configurations in sugars. |
|
|
|
What are the Cahn-Ingold-Prelog rules for assigning priority?
|
Start with the atoms directly bonded to the chiral carbon.
The atom with the higher MW has greater priority. It is assigned the number 1. The lowest priority is assigned the number 4. The movement goes from 1 to 2 to 3, and if it is counterclockwise, it's S, if it's clockwise it's R. If atoms are the same, look at the group of atoms attached to the atoms that are the same. The group with the higher MW has greater priority or the group with more bonds to the high MW species wins. For example, -CHO will have higher priority than -CH2OH because the carbon has a double bond to oxygen. Double bonds are counted twice in groups and triple bonds are counted three times in groups. Another example is, -CH(OH)2 will have higher priority than -CH2OH because the diol has 2 oxygens while the alcohol only has 1. What about -CH(OH)2 vs. CH2F? Ans: It doesn't matter how many oxygens there are, because fluorine has greater molecular weight. So fluorine has higher priority. |
|
|
|
What are the Steps in assigning (R) and (S)?
|
Steps in assigning (R) and (S):
a. Is the carbon center chiral? For our molecule, the answer is yes because 4 different groups are attached to the carbon atom. b. Assign priorities according to the Cahn-Ingold-Prelog rules c. Turn the molecule such that the lowest priority group at the back. d. Rotate from the 1st to 2nd to 3rd priority group like a steering wheel. It's (R) if you end up turning right, and it's (S) if you end up turning left. note: if the highest priority group is shifted to the back, assign the R or S, but then switch it! The lowest priority group has to be facing the back, not the highest. So watch out for where the highest priority group is facing. |
|
|
|
What are the conventions for writing R and S forms?
|
If only 1 chiral center
(R/S)-molecule, where R/S is the absolute configuration and molecule is the name of the compound. For example, (R)-2-hydroxyl-propanal. If more than 1 chiral center (#R/S, #R/S)-molecule, where # is the carbon number (in ascending order), R/S is the absolute configuration, and molecule is the name of the compound. For example, (2R,3S)-2,3,4-hydroxyl-butanal. |
|
|
|
What are the conventions for writing E and Z forms?
|
If only 1 double bond
(E/Z)-molecule, where E/Z is the geometric configuration across the double bond, and molecule is the name of the compound. For example, (Z)-2-chloro-2-butene. (see geometric isomer figure) If more than 1 double bond (#E/Z, #E/Z)-molecule, where # is the carbon number (the smaller number in the double bond, in ascending order), and molecule is the name of the compound. By the smaller number in the double bond, we would use the carbon with the number 2 and not the carbon with the number 3 of the double bond. So the name of the molecule would be 2E,4Z instead of 3E,5Z. |
|
|
|
What are racemic mixtures?
|
Racemic mixtures contain equal amounts of both enantiomers. Another name for racemic mixtures is racemate.
Racemic mixtures do not rotate polarized light, so they are optically inactive. |
|
|
|
What is the nomenclature for hydrocarbons?
|

After Decane, there is Undecane (11), Dodecane (12), Tridecane (13), Tetradecane (14), and so forth for eleven membered alkanes upwards.
|
|
|
|
Explain the concept of ring strain in cyclic compounds.
|
Cyclopropane has the highest ring strain. |
|
|
|
What kinds of strain does ring strain consist of?
|
Ring strain consists of Angle (Baeyer) strain and Torsional strain. Angle (Baeyer) strain is caused by deviation from the ideal sp3 tetrahedral bond angle of 109.5°. As an example of angle strain, take cyclic alkanes, in which each carbon is equally bonded two carbons and two hydrogens. Since each of the four bonds of a carbon is equivalent, it is sp3 hybridized and ideally should have cos−1(−1/3) ≈ 109.5° (the angle that maximizes the distance between atoms) bond angles. Due to the limitations of cyclic structure, however, the ideal angle is only achieved in a six carbon ring - cyclohexane, and only when it is in its chair conformation. For other cyclic alkanes, the bond angles deviate from ideal. In cyclopropanes (3 carbons) and cyclobutanes (4 carbons) the C-C bonds will be 60° and ~90° respectively. Torsional strain is caused by the molecule having eclipsed conformations instead of staggered ones. Cyclopropane has both angle (Baeyer) strain and torsional strain. Cyclohexane, in the chair conformation, has no angle (Baeyer) or torsional strain. You'll frequently see people write Bayer strain instead of Baeyer strain. They mean the same thing. |
|
|

What is the nomenclature for alcohols?
|
Prefix: hydroxyl, hydroxy. Suffix: -ol, alcohol. In SN1 reactions with alcohols - a carbocation intermediate is formed, it is unimolecular (only depends on 1 molecule to react), and the mechanism has two steps. The rate of the SN1 reaction depends on the concentration of the ELECTROPHILE only, not the nucleophile. The nucleophile (could be a halogen) attacks the electrophile (the carbocation with the alcohol attached). For SN2 reactions, the rate determining step is bimolecular (meaninig it depends on two reactants to react) and the reaction occurs in 1 step. SN2 reactions depend on the concentration of both the electrophile (what's being substituted), and the nucleophile (the alcohol). Good nucleophiles are electron-rich, so molecules with a negative charge are usually good nucleophiles. SN1 reactions require acid catalysts like ZnCl2, H2SO4, and HCl because the OH makes a very poor leaving group without heating the alcohol in the presence of concentrated aqueous acid, thus protonating the OH group to OH2+. OH2+ is a great leaving group! The SN2 reaction with an optically active alcohol proceeds with inversion of configuration whereas the SN1 reaction produces racemization. In an SN1, the nucleophile attacks the planar carbocation. Since there is an equally probability of attack on each face there will be a loss of stereochemistry at the reactive center as both products will be observed. Alcohols react with hydrogen halides by nucleophilic substitution. The OH group is replaced by a halogen; water is the byproduct. In the reaction mechanism, the first step involves formation of an oxonium ion by the Lewis acid-base reaction of the hydrogen ion of the hydrogen halide and alcohol oxygen. The rest of the reaction occurs by one of the nucleophilic substitution mechanisms depending on structure of the alcohol. Tertiary and secondary alcohols react by the SN1 mechanism because they can form relatively stable intermediate carbocations; primary alcohols react by the SN2 mechanism that does not require a carbocation. The relative rates of reaction are 3'>2'>1'.
|
|
|

What factors do SN1 and SN2 substitution reactions depend on for alcohols? |
One method of making alkyl halides is by the reaction of alcohols with hydrogen halides via nucleophilic substitution. Factors that favor SN1: stable carbocation, tertiary carbon center (from tertiary alcohol), protic solvent, and steric hindrance. Protic solvents are solvents which can donate H+, whereas aprotic solvents cannot donate H+. Factors that favor SN2: unstable carbocation, primary carbon center (from primary alcohol), aprotic (but polar) solvent, and the least steric hindrance. All substitution reactions need a good leaving group. SN1 = unimolecular reaction, intermediate carbocation formed. Occurs in two steps. SN2 = bimolecular reaction, passes through transition state. Occurs in one step. The oxygen atom of an alcohol is nucleophilic and is therefore prone to attack by electrophiles. The resulting intermediate then loses a proton to a base, giving the substitution product. The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides or, under strongly acidic conditions, with secondary or tertiary alcohols. With primary alkyl halides, the alternative SN2 reaction occurs. The SN2 reaction (also known as bimolecular nucleophilic substitution or as backside attack) is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step. Since two reacting species are involved in the slow, rate-determining step of the reaction, this leads to the name bimolecular nucleophilic substitution, or SN2. SN1 and E1 reactions occur under the same conditions, similar to SN2 and E2 reactions, it's just that instead of a substitution, the E1 and E2 reactions eliminate the OH and create a double bond. Protic solvent's don't work for SN2 substitutions because the nucleophile is already surrounded by protons, so it doesn't need to attack and it's stabilized. Protic solvents facilitate carbocation formation because it stabilizes the leaving group in SN1 substitution. Aprotic solvents are polar, but they don't do hydrogen bonding. Aprotic solvents stabilize the transition state, but they don't stabilize the leaving group or nucleophile where carbocations would be formed. |
|
|

How are alcohols oxidized?
|
KMnO4, Na2Cr2O7, H2Cr2O7, and CrO3 will oxidize primary alcohols to carboxylic acids and secondary alcohols to ketones, but PCC will only oxidize a primary alcohol to the aldehyde. Tertiary alcohols do not oxidize. The oxidation of an alcohol involves the loss of one or more (alpha) hydrogens from the carbon-bearing OH group. The type product formed depends upon how many of these (alpha) hydrogens the alcohol contains -- that is, whether the alcohol is primary, secondary, or tertiary. A primary alcohol contains two (alpha) hydrogens, and can lose one of them to form an aldehyde or both of them to form a carboxylic acid. A secondary alcohol can lose its only (alpha) hydrogen to form a ketone. A tertiary alcohol contains no (alpha) hydrogens and is not oxidized. PCC is also C5H5NH(+)CrO3Cl(–), as shown in the diagram.
|
Alcohols can also be oxidized by the addition of halogens (X2). Br2 is an oxidizing agent. Alcohols can be reduced by the loss of halogens (X2). |
|
|
What are the reactions of alcohol with SOCl2 and PBr3?
|
R-OH + SOCl2 --> R-Cl (by products: SO2 + HCl)
R-OH + PBr3 --> R-Br (by products: H3PO3, R3PO3, HBr) |
|
|
|
How can mesylates and tosylates be prepared?
|
Sulfonates R-SO3- are good leaving groups.
The R can be: Methane, which makes methanesulfonate. Toluene, which makes tosylate. Trifluoromethane, which makes triflate. Mesylates can be prepared by reacting an alcohol (R-OH) with mesyl chloride (MsCl). Tosylates can be prepared by reacthing an alcohol (R-OH) with tosyl chloride (TsCl). |
|
|
|
What is keto–enol tautomerism? |
Enol form is the one with the alcohol. Keto form is the one with the ketone. Keto form is more stable, it is the predominant form. Carbonyl compounds with a hydrogen on the α-carbon (carbon next to the carbonyl) rapidly equilibrate between the keto form (carbonyl) and the enol form (C=C bond with OH). This process formally involves the movement of a proton from the α-carbon to the oxygen and the π-bond from C-O to C-C. Note, that this is not resonance since an atom is moving. This type of isomerization is called tautomerization, which is any rapid equilibrium that involves movement of one or more atoms (usually protons). The α-carbon of an enol is nucleophilic. The α-position will react with electrophiles to give α-substituted carbonyl derivatives. Tautomers are structural isomers. Resonance forms are representations of contributors to a single structure and the only thing shifting around are electrons. Here, hydrogens are shifting around. So if you have a question about resonance structures, only pick those structures that shift charges. Resonance is the appearance of delocalized electrons within certain molecules or polyatomic ions, so that the bonding cannot be expressed by one single Lewis formula. A molecule or ion with such delocalized electrons is represented by several resonance structures. Enol ends with an "ol" so it has an alcohol group. Keto is a ketone group. |
|
|
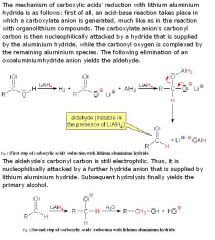
What reduction reaction are COOH involved in?
|
■LiAlH4 + COOH -> alcohol. |
|
|

When does decarboxylation occur for COOH? |
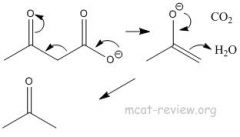
Decarboxylation: occurs for beta-keto acids. Decarboxylation is a chemical reaction which releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids.
|
|
|
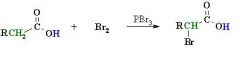
What is the mechanism of the halogenation reaction at α position of carboxylic acids? |
Halogenation: RCOOH + X2 -> halogenation at the alpha carbon (2 position). Reagents most commonly : Br2 and either PCl3, PBr3. Carboxylic acids can be halogenated at the C adjacent to the carboxyl group. This reaction depends on the enol type character of carbonyl compounds. The product of the reaction, an a-bromocarboxylic acid can be converted via substitution reactions to a-hydroxy- or a-amino carboxylic acids. Acyl or acid halides are derivatives of carboxylic acids. They have the general formula RCOX. The CO is a carbonyl (C=O). The mechanism involves the formation of acid bromide with the PBr3. Because the acid bromide is much more electrophilic than a carboxylic acid, the enol form is formed more easily. The enol then reacts with bromine to give the α-brominated acid bromide. Treatment with water gives the α-bromoacid. Note that any nucleophile could be used to react with the acid bromide (i.e. an alcohol to give an ester or an amine to give an amide). |
|
|
|
What are the general principles of H bonding of COOH molecules? |
•H bonding: COOH has high boiling point because of H bonding. |
|
|
|
What are the rules of precedence for Cahn-Ingold-Prelog?
|
Ligands of the higher atomic number precede those with lower ones, e.g. Br precedes Cl (Br>Cl).
For ligands with the same type of atoms linked to the center C, the precedence is determined based on the atomic numbers of ligands in the next sphere, e.g. ligand with C-O sequence precedes C-C. If no difference is detected, the determination is based on the distinction in the next spheres, and search is continued until the difference is detected. The coordination number of non-hydrogen atoms is assumed to be 4, i.e. atoms bonded with multiple bonds are considered to be bonded to multiple atoms, e.g. carbonyl carbon is treated as if it was bonded to two oxygen atoms, and carboxyl carbon as if it was bonded to three oxygens (these are then called phantom atoms). |
|
|
|
What is halogenation of alkanes?
|
Substitution reactions with halogens or halogenation. Alkane + halogen + free radical initiator → alkyl halide. CH4 + Cl2 + energy ——> CH3Cl + HCl. Free radical initiators = hν (UV light) or peroxides. Substitution occurs via a free radical mechanism. Halogenation is the replacement of one or more hydrogen atoms in an organic compound by a halogen (fluorine, chlorine, bromine or iodine). Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in which a C-H bond is broken and a new C-X bond is formed. The reactivity of the halogens decreases in the following order: F2 > Cl2 > Br2 > I2. Energy input in the form of heat or light is necessary to initiate these halogenations. Halogenation reactions may be conducted in either the gaseous or liquid phase. In liquid phase halogenations radical initiators such as peroxides facilitate the reaction. The most plausible mechanism for halogenation is a chain reaction involving neutral intermediates such as free radicals or atoms. A radical is an atomic or molecular species having an unpaired, or odd, electron. Between chlorination and bromination, the bromine is more selective than the chlorine. Bromination is more endothermic because it is more selective.
|
Halogenation is an exothermic process. Alkyl radicals exhibit trigonal planer geometry. The stability of the alkyl radical follows the same order as carbocation stability: 3°>2°>1°>methyl. Keep in mind that if there are more primary hydrogens to react with than tertiary hydrogens, the radicals may react with a primary carbon instead of what we would usually predict: a tertiary carbon. |
|
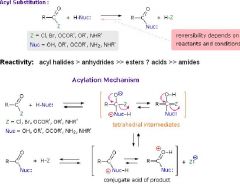
What are good leaving groups? |
A leaving group , LG, is an atom (or a group of atoms) that is displaced as stable species taking with it the bonding electrons. Typically the leaving group is an anion (e.g. Cl-) or a neutral molecule (e.g. H2O). The better the leaving group, the more likely it is to depart. A "good" leaving group can be recognized as being the conjugate base of a strong acid. For acidity, the more stable A- is, then the more the equilibrium will favour dissociation, and release of protons meaning that HA is more acidic. For the leaving group, the more stable LG- is, the more it favours "leaving". If you have an alkyl halide, and it is reacted with sodium hydroxide, the alkyl with the best leaving group will react the fastest. Since I- is one of the best leaving groups, t-butyl iodide will react faster than t-butyl chloride, t-butyl fluoride, and t-butyl bromide. This is different from the reactivity of halogens in halogenation, in which fluoride reacts the fastest. The difference is that the halogens are LEAVING the alkyl in the reaction with sodium hydroxide, where as the halogens are substituted to the molecule. SN1 and SN2 reactions with alcohols work great in acidic solutions because the acid protonates the OH group and make it an OH2+ group, which makes it a great leaving group.
I>Br>Cl in terms of leaving groups. The better the leaving group, the stronger the conjugate acid (I- versus Br- and Cl-). |
|
|
|
What are 6 things to remember about SN1 versus SN2?
|

|
|
|
|
How can alkyl halides be created from alcohols?
|
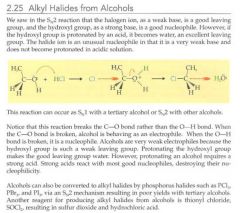
|
|

