![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
69 Cards in this Set
- Front
- Back
|
EQ - Max number of electrons that can fill a subshell |
4l + 2
|
|
|
EQ - Max number of electrons allowed in single energy level in terms of n
|
2n^2
|
|
|
Diamagnetic vs Paramagnetic
|
Diamagnetic - All electrons are spin-paired; unaffected by magnetic fields
Paramagnetic - Has unpaired electrons - affected by magnetic fields |
|
|
Heisenberg Uncertainty Principle:
Cannot know ________ + _______ |
momentum and position
|
|
|
Hund's Rule vs Pauli Exclusion Principle
|
Hund - electrons fill empty orbitals first
Pauli - Each orbital contains two electrons of opposite spin |
|
|
Malleability vs ductility
|
Malleable - mold into shapes
Ductile - drawn into wire |
|
|
Type of bond formed between Lewis Acid and Base
|
Coordinate Covalent Bond
Ex - Ammonia has 3 polar covalent bonds Ammonium has 1 more, which is coordinate covalent. |
|
|
Disperion Forces
|
Types of van der Waals force that occurs among all bonded atoms due to unequal sharing of electrons
(Dipole-dipole is another type of vdW. Noble gases only have dispersion forces) |
|
|
Molarity Molality Normality
|
Molarity - Moles/L
Molality - mol solute/ KILOGRAM solvent Normality - Equivalents / L |
|
|
Molecule vs Compound
|
All compounds are molecules but not all molecules are compounds
Molecules are discrete , isolateable units. Ex - Ionic compounds are bonded interweavingly. No discrete units. So graphite consists of a bunch of carbons bonded in a random pattern. |
|
|
Ionic compounds are not molecules
|
THEY ARE NOT FREAKING MOLECULES OKAY YOU IDIOT???
THEY ARE COMPOUNDS! They don't have a molecular weight or are made from molecules. |
|
|
D - Gram Equivalent Weight
|
Weight that would release one acid equivalent
So H2SO4 has a gram equivalent weight that's half of its molecular weight. Moron. |
|
|
Exponents of rate law vs equilibrium expression
|
Equilibrium - exponents are the coefficients of each component
Rate law - exponents are the freaking rate law, idiot. |
|
|
EQ - Concentration of radioactive decay
|
Radioactive decay is FIRST ORDER
[A] = [Ao]e^(-kt) |
|
|
Energy relationship of reactants, products, and transition sate
|
Transition state ALWAYS has the highest.
Endothermic - reactants have lower Exothermic - reactants have higher. Since you know, energy is being lost. Duhhhhh |
|
|
Relationship - Law of Mass Action and Reaction Quotient
|
Law of Mass Action = Rate Law = reactants/products
Reaction quotient is a relative measure of how far a reaction is toward equilibrium: Q < K - heading there Q > K - exceeded Q = K - reached it! |
|
|
Gibbs - Conditions for an endothermic, spontaneous reaction
|
H is positive - because it's endothermic, duh
G is negative - because it's spontaneous G = H - T(S) In order to make G negative, T(S) has to be positive. T is always positive unless we violate the Third Law of Thermodynamics trollolololololol. So S has to be positive. |
|
|
EQ - Solubility Product Constant
|
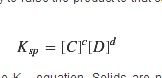
PRODUCTS ONLY
RAISED BY THEIR STUPID COEFFICIENTS AS EXPONENTS SONOFABEACH |
|
|
L - Systems (3)
|
Isolated - doesn't exchange energy or matter,
Closed - Exchanges energy but not matter, like a radiator Open - Exchanges energy and matte |
|
|
L - Processes (3)
|
Isothermal - constant temperature, no duh
Adiabatic - no heat exchange Isobaric - constant pressure |
|
|
L/D - State Functions
|
Mneumonic - VG PHEST
Volume Gibbs Pressure Enthalpy Internal ENERGY Entropy Temperature ***** ALSO DENSITY State Functions - independent of path taken *not necessarily independent of each other |
|
|
Heat vs Temperature
|
Heat is the transfer of energy
Temperature is a measure of particle average kinetic energy |
|
|
EQ - standard free energy using equilibrium constant
|
G = -RTln(Keq) THIS IS AT EQUILIBRIUM
Afterwards, ya gotta use: G = Go + RTlnQ Which turns into: G = RTln(Q/Keq) |
|
|
EQ - energy in relation to heat and work
|
U = Q - W
Energy is heat minus work HEAT MINUS WORK DAMMIT HEAT MINUS WORK |
|
|
EQ - work in relation to volume and pressure
|
W = -P(delta)V
|
|
|
Adiabatic in terms of pressure and volume
|
Volume changes that occur without a significant loss of heat (through manipulation of pressure for example)
Think PV curve in hot->cool transitions |
|
|
Different variables that influence BP and MP
|
Branching decreases BP
Symmetry increases MP, decreases BP linear compounds have higher BP and MP Super branched compounds will have higher MP but lower BP |
|
|
Effect of branching on heat of reaction
|
Branching DECREASES
Makes it harder to do, so more heat input is needed. |
|
|
e
|
2.7
|
|
|
Radical 3 and 2
|
1.7 and 1.4
|
|
|
R (gas constant)
|
8.314 J/mol K
.082 Latm/molK |
|
|
density of water
|
1000kg/m^3
1g/cm^3 |
|
|
Difference between adiabatic and isothermal
|
THEY ARE MUTUALLY EXCLUSIVE IF YOU EVER SAY THAT THEY CAN BOTH HAPPEN IN ONE PROCESS YOU'RE A DUMBHEAD
Adiabatic - no heat transfer Isothermal - constant temperature (requires transfer of heat around) |
|
|
Endergonic/Exergonic vs Endothermic/Exothermic
|
Exo/Endothermic are relative to enthalpy only
Ender/exergonic are relative to spontaneity. Endergonic is nonspontaneous storage of energy. Exergonic is spontaneous release of energy |
|
|
Spontaneity
Q < Keq |
Spontaneous
Think G = RTln(Q/Keq), we want a fraction |
|
|
Spontaneity
Q > Keq |
Nonspontaneous
Think G = RTln(Q/Keq) we want a fraction |
|
|
To measure heat capacity/specific heat you use
|
A calorimeter
Heat capacity - amount of energy/temp Specific heat - energy to raise temperature of 1g by 1 Celsius |
|
|
Laws of thermodynamics expressed as equations
|
1. E(system) + E(surroundings) = E(universe)
2. S(system + S(surroundings) = S(universe) 3. S(universe) = o at T = 0K 0th - thermal equilibrium |
|
|
EQ - average kinetic energy of a gas particle
|
KE = .5mv^2 = 3/2kT
|
|
|
Root-mean-square speed of gas
|
u(rms) = radical(3RT/M)
|
|
|
When do you have to consider van't Hoff Factors?
|
In calculations involving colligative properties:
FBD - miK BPE - miK OP - iMRT |
|
|
Alloy vs Pure Metal MP/FP
|
LOWER - pure metals have much stronger metallic bonding
|
|
|
Solubility Rules
|
SODIUM AND NITRATE SALTS ARE ALL SOLUBLE
1. Salts of Alkali Metals - soluble 2. Salts of Ammonium - soluble 3. Salts of Cl, Br, I soluble (EXCEPT Ag+, Pb2+, Hg2(2+)) 4. Salts of Sulfate are soluble, except Pb2+, Ba2+, Sr2+, and Ca2+ 5. All metal oxides except ones with alkali metals (and CaO, SrO, BaO) are INSOLUBLE 6. All hydroxides are INSOLUBLE except Ca2+, Sr2+ and Ba2+ 7. Carboantes, phosphates, sulfides, and sulfites are all INSOLUBLE except alkali and ammonium |
|
|
L - UNITS OF CONCENTRATION
|
Mole Fraction - moles of compound divided by total number of moles of all species within a system
- VPD and partial pressures Molarity - Moles/liter(solution) Molality - moles/kg(solvent) Normality - Equivalents/Liter(solution) |
|
|
L - General Ksp values for salts
**************************************************************THIS WAS ON A PRACTICE TEST YOU HO |
MX3 - 27x^4
MX2 - 4x^3 MX - X^2 X = molar solubility, mols/L dissolved |
|
|
What the Aych Eee Double Hockey Sticks is I.P.??
|
Ion Product. The same as Q for equilibrium constant. In relation to Ksp
If IP If IP=Ksp it's saturated If IP>Ksp, solution is unsaturated, precipitation REMEMBER: Q < K - heading there Q > K - exceeded Q = K - reached it! |
|
|
Most dissolutions are _____________- EXCEPT
|
Most dissolutions are endothermic
EXCEPT dissolution of gas into liquid (exothermic) |
|
|
D - solution
|
homogenous mixtures of two or more substances that combine to form a single phase
* Solutions are mixture,s but not all mixtures are solutions |
|
|
Raoult's Law
|
Ideal solution behavior is observed when solute-solute, solvent-solvent, and solute-solvent interactions are very similar
|
|
|
How to determine charge density
|
1.Calculate charge of ions (highest total charge, highest charge density)
2. Smallest size, highest charge density |
|
|
Mixtures that have higher vapor pressure than predicted by Raoult's Law
|
Have stronger solvent-solvent/solute-solute interactions than solvent-solute
|
|
|
Relationship between solubility and atmospheric pressure
|
THEY IS DIRECTLY PROPORTIONAL POOBUTT
|
|
|
Important Properties of logarithms
|
log x^n = n log x
log10^x = x |
|
|
Strong Acids
|
HCl
HBr HI H2SO4 HNO3 HClO4 |
|
|
Strong Bases
|
NaOH, KOH
other crap |
|
|
Gram Equivalent Weight
|
Grams of substance divided equivalents
|
|
|
Threshold for Ka acidity
|
if Ka > 10^-7
ACID |
|
|
Characteristics of Concentration Cells
|
Spontaneous redox reactions
Generate Current Supply energy *** current is dependent on ion concentration gradient, not difference in reduction potential. Go and Eo are both 0, because current ceases when concentrations of ion are equal |
|
|
Compare Galvanic, Concentration, and Electrolytic Cells
|
Galvanic - Spontaneous, current, energy, G-, E+
Concentration - Spontaneous, current, energy, G = 0, E = 0. Current is dependent on ion concentration gradient Electrolytic - Nonspontaneous, requires external voltage, consumes energy. G+, E- |
|
|
EQ - Relationship between emf and equilibrium constant
|
G = -nFE = -RTlnK
Holy crap that's confusing |
|
|
Characteristics of Concentration Cells
|
Spontaneous redox reactions
Generate Current Supply energy *** current is dependent on ion concentration gradient, not difference in reduction potential. Go and Eo are both 0, because current ceases when concentrations of ion are equal |
|
|
Compare Galvanic, Concentration, and Electrolytic Cells
|
Galvanic - Spontaneous, current, energy, G-, E+
Concentration - Spontaneous, current, energy, G = 0, E = 0. Current is dependent on ion concentration gradient Electrolytic - Nonspontaneous, requires external voltage, consumes energy. G+, E- |
|
|
Overall potential of a spontenous system
|
POSITIVE
|
|
|
What is E^o
|
REDUCTION POTENTIAL. WHICH IS USED TO CALCULATE STANDARD ELECTROMOTIVE FORCE (EMF)
Metals have a very negative one, because they like to be oxidized |
|
|
A species with higher reduction potential...
|
Is more likely to BE REDUCED, and DO THE OXIDIZING
|
|
|
Redox of metal with oxygen
|
METAL IS OXIDIZED (donates electrons)
|
|
|
Is it possible for halogens to have a positive oxidation number?
|
Yes apparently. Crap... example is Bleach, NaClO
|
|
|
*** High Yield ***
When you have intermediates in your rate law (slow step) what do you do? |
Use prior steps to solve for concentrations, and substitute
|
|
|
ite-ous ate-ic |
duh. |

