![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
If the solution has pH=8 and an acid is add of pKa=7, what is the ratio of protonated to unprotonated acid?
|
pH - pkA = 1
[A-]: [HA] = 10^1 : 1 so 10:1 will be deprotonated:protonated |
|
|
In a nearsighted person:
1. what can the person not focus on? 2. where does the image form? 3. What type of lens is needed? |
Distant objects
Before the retina (Inside the eye) Diverging lens |
|
|
In a farsighted or presbyopia (can't contract muscle well)person:
1. what can the person not focus on? 2. where does the image form? 3. What type of lens is needed? |
Close objects
after the retina Converging |
|
|
What kind of bond forms after dehydration of an alcohol?
What happens if you add Br2 and C(Cl)4 to the product? |
It will create an alkene
The C=C will cause the red color of bromine to disappear. |
|
|
Cyclohexane
|
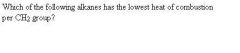
Cyclopropane
Cyclobutane Cyclohexane Cycloheptane |
|
|
What is the best way to separate two solutions with different polarities?
|
Heat them very slowly, the H bonds will keep the more polar one in liquid phase.
Cooling column has no effect. |
|
|
What is the definition of codominant allele? Give example
|
When a an allele is not dominant over another. Example is ABO blood type, both A and B are dominant over O, but the person produces both A and B antibodies, therefore they are codominant
|
|
|
What is the definition of incomplete dominance? Give example
|
When no allele is dominant over another, gives a dosage effect. Example is red and white snapdragons yield pink progeny.
|
|
|
What is Epistasis?
|
Epistasis is the term applied when one gene interferes with the expression of another.
Yield 9:7 progeny for 2 alleles versus 9:3:3:1 |
|
|
Hydrogen goes from oxidation state +1 to 0 in H2
|

Redox
|
|
|
If a solution is 98% H2SO4 by weight, what is the % of HSO4- ions?
|

[] of dirpotic acid
|
|
|
1+1=2 second order rxn
remember that rate laws do not necessarily have their coefficients as exponential functions, must be determined experimentally unless given |
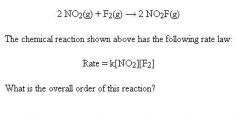
Rate Law
|
|
|
+185 C
|
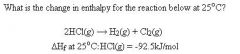
Heat of Formation
|
|

Lens
|

1/3
|
|
|
What is the definition of out of phase?
|
When waves do not reach the same point at the same time, even if they are the same frequency and wavelength.
Like 2 runners running the same speed on the same track but at different starting points. |
|
|
When the MCAT says beta decay, which do they mean?
|
B- decay
|
|
|
What is the only decay in which the element doesn't change and why?
|
Gamma decay, because the charge doesn't change. All other decays lose or gain a charge (-2 for alpha)
|
|
|
As electroaffinity becomes more negative, what trend increases?
What trend becomes more negative? |
Electron ionization energy increases because if a atom is has a high affinity for electrons (more negative=more affinity), it will take increased energy to break e away
EA is the amount of energy liberated if an electron is added, so if it takes a lot of energy to pull an e away from F- (ionization energy), there is a large amount of energy liberated when that e is replaced (electron affinity) Bottom line Ionization energy is inversely proportional to electron affinity. Note also nobel gases have no EA because they are stable |
|
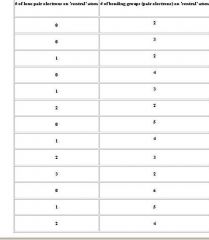
Fill in table
|
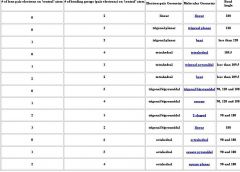
VSPER
|
|
|
Name all shape and geometry of 2 binding groups
|

VSPER
|
|
|
Name all shape and geometry of 3 binding groups
|

Vsper
|
|
|
Name all shape and geometry of 4 binding groups
|

vsper
|
|
|
Name all shape and geometry of 5 binding groups
|
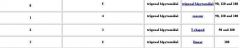
vsper
|
|
|
Name all shape and geometry of 6 binding groups
|

vsper
|
|
|
When springs of equal K are on oppostite sides of a mass, what is the effective spring constant of the system
|
2K
Springs on opposite sides can be summed F=- K x +(-K)(-x) =2Kx |
|
|
When is a solid body in translational equilibrium?
When is it in rotational equilibrium? |
A solid body is in translational equilibrium when the sum of its external forces = 0
A solid body is in rotational equilibrium when the sum of its external forces AND torques = 0 |
|
|
How do you determine rate laws?
When can it be assumed? Give general formula What does it always involve and what does it usually involve? |
Rate law can only be determined experimentally unless it is an elementary reaction (either first order x=1 for monatomic rxn, or second order for biatomic reaction x+y=1+1=2)
Generally you use: rate = k [A]^x [B]y Usually only depends on reactants, but can include products. Only uses molecules involved in rate determining step |
|
|
Give Arrhenius eqn.
What are 2 factors that will increase the rate of the rxn? |
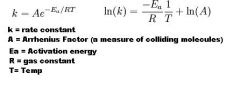
From the second eqn:
Rxn rate can increase by 1. Adding catalyst to lower Ea 2. Increasing temp |
|
|
What are common strong bases?
Common weak bases? |
Common Strong Bases
Group I hydroxides LiOH or NaOH and metal amides NaNH2 Common weak bases Group II hydroxides Ca(OH)2 |
|
|
How do you calculate the pH of a strong acid?
|
pH = - log [H+]
so [H+] = 10^(-pH) |
|
|
How do you determine pH of weak aci?
|
For a weak acid you need to calculate the Ka by determining the ratio of dissociated ions
Set up eqn: Ka = [H+][CN-] / [HCN] Ka = X*X /.2-x Assume x in .2-x is neglidible because it is a small amout thus Ka = x^2/.2 You will need other information to solve for x but this is the principle used |
|
|
What eqn is used for making for measure pH of a buffer solution of weak acid? How would the eqn change for a weak base?
|
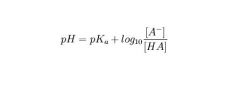
Henderson-Hasselbalch
For a base: pOH = pKb + log [HA]/[A-] |
|
|
What is an assumption of buffer range?
|
It's pH is +/- 1 of the pKa
|

