![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
43 Cards in this Set
- Front
- Back
|
Hydrophobic
|
Not interacting readily with water. Hydrophobic compounds are typically nonpolar compounds that lack charged or electronegative atoms and often contain many C – C and C – H bonds.
-molecules move away from water (i.e. lipids) |
|
|
Hydrophilic
|
Interacting readily with water. Hydrophilic compounds are typically polar compounds containing charged or electronegative atoms.
-molecules dissolve easily in water because their negatively charged ends attract the positively hydrogens of water, and their positively charged ends attract the negatively charged oxygen of water. -Water molecules solvate the hydrophilic molecule |
|
|
Hydrolysis
|
A chemical reaction in which a molecule is split into smaller molecules by reacting with water. In biology, most hydrolysis reactions involved the splitting of polymers into monomers.
|
|
|
Dehydration synthesis (aka condensation reaction)
|
A chemical reaction in which two molecules are joined covalently with the removal of an -OH from one and a -H from another to form water.
|
|
|
Lipid
|
Any organic substance that does not dissolve in water, but dissolves well in nonpolar organic solvents. Lipids include fats, oils , phospholipids, and waxes.
MAJOR FUNCTIONS OF LIPIDS -Phospholipids: structural component of membranes -Triacylglycerols: store metabolic energy, provide thermal insulation and padding -Steroids: regulate metabolic activities -Fatty acids: some serve as local hormones (eicosanoids) -fats are lipids, but not all lipids are fats (e.g. steroids & terpenes) |
|
|
Fatty acids
|

A lipid consisting of a hydrocarbon chain bonded to a carboxyl group (-COOH) at one end. Used by many organisms to store chemical energy; a major component of animal and plant fats.
-There is usually an even number of carbons, with the maximum number of carbons in humans being 24. -Short-chained fatty acids are slightly water soluble, while longer-chained fatty acids are more than likely non-water soluble. |
|
|
Saturated fatty acids
|
Referring to fats and fatty acids in which all the carbon-carbon bonds are single bonds. Such fats have relatively high melting points.
|
|
|
Unsaturated fatty acids
|
Referring to fats and fatty acids in which at least one carbon-carbon bond is a double bond. Double bonds produce kinks in the fatty acid chains and decreased the compounds melting point and energy storage potential.
|
|
|
Triacylglycerols (aka triglycerides or fat)
|
A lipid consisting of three fatty acid molecules joined by ester linkages to a glycerol molecules.
|
|
|
Adipocytes
|
Specialized cells whose cytoplasm contains almost nothing but triglycerides.
|
|
|
Phospholipids
|

A class of lipid having a hydrophilic head (a phosphate group) and a hydrophobic tail (one or more fatty acids). Major components of the plasma membrane and organelle membranes.
-amphipathic molecules |
|
|
Amphipathic
|
Containing hydrophilic and hydrophobic elements
|
|
|
Glycolipid
|

Any lipid molecule that is covalently bonded to a carbohydrate group.
-Glycolipids are amphipathic -found in abundance in the membranes of myelinated cells composing the human nervous system. |
|
|
Steroids
|
A class of lipid with a characteristic ring structure.
-includes hormones vitamin D and cholestrol |
|
|
Terpenes
|
organic hydrocarbons or hydrocarbon derivatives constructed from recurring isoprene units.
-includes vitamin A, a vitamin important for vision |
|
|
Lipoprotein
|
A lipid-protein aggregate that
serves to carry water-insoluble lipids in the btood The protein coniponent alone is an apolipoprotein. -major classes include: chylomicrons, very low density lipoproteins (VLDL), low density lipoproteins (LDL), and high density lipoproteins (HDL) |
|
|
Proteins
|
A macromolecule consisting of one or more polypeptide chain composed of 50 or more amino acids linked together. Each protein has a unique sequence of amino acids and, in its native state, a characteristic three-dimensional shape.
-when you see nitrogen in the MCAT BS section, think protein (which can be denatured) |
|
|
Primary structure
|
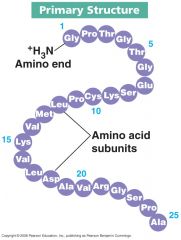
The sequence of amino acids in a peptide or protein; also the sequence of nucleotides in a nucleic acid.
-linked by covalent bonds -determines structure and function |
|
|
Secondary structure
|
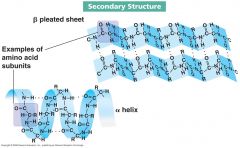
In proteins, localized folding of a polypeptide chain into regular structures (e.g. a-helix and B-pleated sheet) stabilized by hydrogen bonding between atoms of the backbone. In nucleic acids, elements of structure (e.g. helices and hairpins) stabilized by hydrogen bonding and other interactions between complementary bases.
|
|
|
Tertiary structure
|
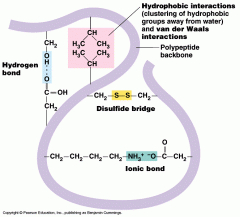
The overall three-dimensional shape of a single polypeptide chain, resulting from multiple interactions among the amino acid side chains and the peptide backbone.
FIVE FORCES THAT CREATE TERTIARY STRUCTURE: 1. Covalent disulfide bonds between two cysteine amino acids on different parts of the chain; 2. electrostatic (ionic) interactions mostly between acidic and basic side chains; 3. hydrogen bonds; 4. van der Waals forces (weak); 5. hydrophobic side chains pushed away from water (toward the center of the protein). |
|
|
Quaternary structure
|
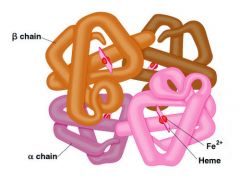
The overall three-dimensional shape of a protein containing two or more polypeptide chains (subunits); determined by the number, relative positions, and interactions of the subunits.
|
|
|
Denaturation
|
For a macromolecule, loss of its three-dimensional structure and biological activity due to breakage of hydrogen bonds and disulfide bonds, usually caused by treatment.
-urea disrupts hydrogen bonds -salt or change in pH disrupts electrostatic bonds -mercaptoethanol disrupts disulfide bonds -organic solvents disrupt hydrophobic forces -heat disrupts all forces |
|
|
Globular protein
|
Soluble proteins with a globular (somewhat rounded) shape.
-function as enzymes (i.e. pepsin), hormones (i.e. insulin), membrane pumps and channels (i.e. Na/K pump and voltage-gated sodium channels), membrane receptors (i.e. nicotinic receptors on a post-synaptic neuron), intercellular and intracellular transport and storage (i.e. hemoglobin and myoglobin), osmotic regulators (i.e. albumin), immune responses (i.e. antibody), etc. |
|
|
Structural proteins
|
-made from long polymers
-maintain and add strength to cellular and matrix structures -i.e. collagen and microtubules |
|
|
Glycoprotein
|
Any protein with one or more covalently bonded carbohydrate groups.
-component of cellular plasma membranes |
|
|
Proteoglycans
|
A hybrid macromolecule consisting of a heteropolysaccharide joined to a polypeptide; the polysaccharide is the major component.
-major component of the extracellular matrix |
|
|
Cytochromes
|
Heme proteins serving as electron carriers in respiration, photosynthesis, and other oxidation-reduction reactions.
|
|
|
Carbohydrates
|
Any of a class of molecules that contain a carbonyl group, several hydroxyl groups, and several to many carbon-hydrogen bonds.
-Empirical formula: CH2O -Glucose accounts for 80% of the carbohydrates absorbed by humans -Essentially all digested carbohydrates reaching body cells have been converted to glucose by the liver or enterocytes -In the absence of insulin, only neural and hepatic cells are capable of absorbing sufficient amounts of glucose via the facilitated transport system |
|
|
Glycogen
|
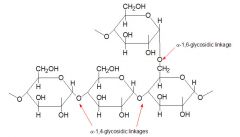
A white amorphous tasteless polysaccharide (C6H10O5)x that is the principal form in which glucose is stored in animal tissues and especially muscle and liver tissue
-contains a-(1-4) linkages |
|
|
Starch
|
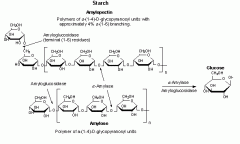
A mixture of two storage polysaccharides, amylose and amylopectin, both formed from a-glucose monomers. Amylopectin is branched, and amylose is unbranched. The major form of stored carbohydrate in plants.
・ All forms of starch contain α-(1,4) linkages ・ Amylose is an unbranched version of starch with only α-(1,4) glycosidic linkages ・ Amylopectin is a branched version of starch with the same α-(1,4) glycosidic linkages, but branching occurs when glycosidic linkages form between carbon 1 of a glucose monomer on one strand and carbon 6 of a glucose monomer on another strand. Branches occur in about one out of every 30 monomers. |
|
|
Cellulose
|
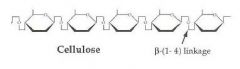
A structural polysaccharide composed of B-glucose monomers joined by B-1,4-glycosidic linkages. Found in the cell wall of algae, plants, bacteria, fungi, and some other groups.
-The beta linkages are digested only in some animals because they contain a bacteria in their digestive systems that release an enzyme to digest the beta linkages |
|
|
Nucleotides
|
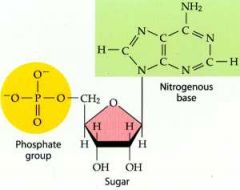
A molecule consisting of a five-carbon sugar (ribose or deoxyribose), a phosphate group, and one of several nitrogen-containing bases. DNA and RNA are polymers of nucleotides containing deoxyribose (deoxyribonucleotides) and ribose (ribonucleotides), respectively. Equivalent to a nucleoside plus on phosphate group.
|
|
|
Nucleoside
|
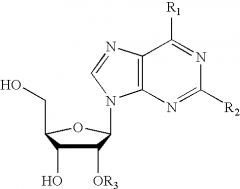
A molecule consisting of a five-carbon sugar (ribose or deoxyribose) and one of several nitrogen-containing bases.
|
|
|
Minerals
|
Dissolved inorganic ions inside and outside the cell.
-create electrochemical gradients -can combine and solidify to give strength to a matrix (i.e. hdroxyapatite) -act as cofactors assisting enzyme or protein functions |
|
|
Enzymes
|
A protein catalyst used by living organisms to speed up and control biological reactions.
-lowers the energy of activation for a reaction therby increasing the rate -are not consumed nor permanently altered by the reactions which they catalyze -DO NOT alter equilibrium -increase temp, reaction increases until denatured -cofactor (non protein that helps enzyme) are metal ions/minerals and coenzymes. -coenzymes are cosubstrates (reversibly bind to enzyme ex: ATP) or prothetic groups (organic; ex: heme) |
|
|
Substrate
|
(1) A reactant that interacts with an enzyme in a chemical reaction.
(2) A surface on which a cell or organism sits. |
|
|
Active site
|
The portion of an enzyme molecule where substrates (reactant molecules) bind and react.
|
|
|
Enzyme-substrate complex
|
The enzyme bound to the substrate
|
|
|
Cofactor
|
A metal ion or small organic compound that is required for an enzyme to function normally. May be bound tightly to an enzyme or associate with it transiently during catalysis.
|
|
|
Coenzyme
|
A small organic molecule that is a required cofactor for an enzyme-catalyzed reaction. Often donates or receives electrons or functional groups during the reaction.
|
|
|
Irreversible inhibition
|
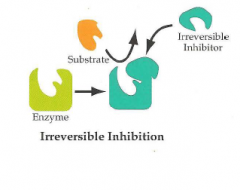
-Bind covalently to disrupt enzymatic function
-A few bind noncovalently |
|
|
Noncompetitive inhibition
|
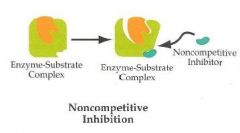
-Bind noncovalently to an enzyme at a site other than the active site
-Lowers Vmax -Km remains the same -Doesn't resemble the substrate |
|
|
Competitive inhibition
|
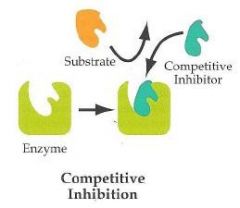
-Bind reversibly with noncovalent bonds
-Raise the apparent Km, but do not change Vmax -Often resemble the substrate |

