![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
180 Cards in this Set
- Front
- Back
|
What is cold-finishing and its characteristics?
|
Processes of rolling, drawing, and forging reduce the cross-section of a metal and strengthen the metal through work-hardening.
|
|
|
What is "turbulence in motion?"
|
Turbulence in motion describes the processes happening internally in a material as it is being cold-worked.
Grains are elongated and compressed, large numbers of dislocations are produced that intersect each other, collisions with alloying elements occur, and many more micro-effects cause the material to gain overall strength. |
|
|
What are key advantages to cold-finishing with respect to dimensional tolerances, cost and surface finish?
|
More precise dimensional tolerance and surface-finishing and cost advantages can be obtained when many small parts are required for production.
|
|
|
What are disadvantages to cold-finishing?
|
lower ductility
greater instability during machining ops higher cost |
|
|
If the stress required for plastic flow in a metal is very low, how will this affect the materials ability to work harden?
|
Materials that require little stress to deform and also do not become overly-brittle will have a greater ability to work harden.
|
|
|
What favorable characteristics do carbon steels have that allow them to work harden?
|
Carbon steels demonstrate favorable plastic flow characteristics.
|
|
|
What is hot finishing?
|
Hot-finishing is a process of shaping done above the recrystallization temperature.
|
|
|
What type of materials are almost exclusively produced by a hot finishing process?
|
Structural steels are almost exclusively hot-finished
carbon steels (c-content < 0.25%) |
|
|
Why does it cost less to hot-finish a material if it costs money to raise the materials temperature?
|
It is cheaper to hot-finish (in general) than use machines with large amounts of pressure and the additional processes required for cold-finishing
|
|
|
Why do hot finished materials have better "weldabiity characteristics" than cold finished materials?
|
Cold-finished metals undergo local annealing when the base metal is recrystallized by the filler metal in the weld.
This loss of strength is avoided by using hot-finished metals. |
|
|
How does machining affect cold-worked materials compared to hot-finished materials?
|
Cold-finished materials have a greater instability during machining operations because of the internal stresses built up by the work hardening process.
|
|
|
Why do carbon steels have poor hardenability?
|
They must be quenched extremely fast in order to retain a BCT Martensitic structure.
This extreme quench is not possible in thick sections when the carbon content is too low. |
|
|
What is the min and max C-content required for hardenability?
|
0.2 is the minimum or the quench rate will be unattainable
0.8% is optimal and above 1.0% and hardenability begins to decrease |
|
|
How can carbon steels hardenability be increased?
|
Add carbon up to 0.8%, add alloys such as Cr, Mo, Si, and Ni
|
|
|
What is weldability?
|
ease with which a weld can be made and the soundness of the weld after it is made
|
|
|
What are the primary control factors for weldability?
|
-general chemical composition
-base metal composition -filler metal composition |
|
|
What is HAZ and why is hardenability an important factor to consider before welding?
|
-the HAZ is the heat-affected zone and is the area on the metal directly next to the weld which is affected by the heat of the welding process
-welding of hardenable steels can lead to cracking in the HAZ |
|
|
What constitutes a carbon steel?
|
Steel with up to 2% C and only risidual amts of other elements (except those used for deoxidation)
|
|
|
What constitutes an alloy steel?
|
Steel with up to 1% C content and no more than a total of 5% alloying elements
|
|
|
In a corrosion cell, the anode will:
|
give up electrons and experience material loss over time
|
|
|
What is Bimetallic (galvanic) corrosion?
|
two differenct metals with a potential difference between them
|
|
|
As a corrosion reaction progresses, a cathode will:
|
begin to lose electrons
|
|
|
What are the 5 components of the corrosion cell?
|
1. Anode: Metal that is corroding and undergoing oxidation, it has the more negative potential
2. Cathode: Metal or other electronic conductor whose surface provides the site for the environment to react 3. Electrolyte: Path for ionic conduction, aqueous environment that is in contact with the anode/cathode 4. Electrical Connection: Path for electrons to flow 5. Potential Difference: Difference in the electrical potential between anode and cathode |
|
|
What differentiates tool steels from basic carbon and alloy steels?
|
Manufacturing processes and quality control in refining steel better
|
|
|
What are 4 differences between basic carbon steels and tool steels?
|
1. Tools have best hardenability
2. Tools have best wear resistance 3. Carbon are the least costly 4. Carbon has a ferrite matrix that is the basis for ductility |
|
|
Which of the following tool steels would probably have the most distortion after a quench-hardening treatment?
O A W D |
W would have the most distortion after a quench-hardening treatment
|
|
|
Electrolytic Tough Pitch is produced from:
|
smelting,
oxidation, and electrolytic refining of copper ore |
|
|
What is the difference between cast and wrought?
|
composition
|
|
|
What are the primary two metals in Brass
|
1. Copper
2. Zinc |
|
|
What are the primary two metals in Cupronickels?
|
1. Copper
2. Nickel |
|
|
What are the primary two metals in Bronze?
|
1. Copper
2. Tin |
|
|
What can happen if oxygen and hydrogen combine at elevated temperatures in copper piping?
|
the combination causes the copper piping material to become brittle at temperatures over 700C if the oxygen content >0.4%
|
|
|
Which of these has (1st) the most high-strength copper alloy and (2nd) the most corrosion resistant?
Pure Copper for both Brass and Bronze Be-Cu and Ni-Cu |
Be-Cu is high strength
Ni-Cu has high corrosion resistance |
|
|
Case Study Conclusions: Corrosion of Stainless Steels in Welds
|
PROBLEM
-austenitic stainless steels usually contain small amts of carbon and @ elevated T's, the C precipitates out -the precipitate combines with chrome in the stainless steel to produce chromium carbide -the chromium carbide reduces the protective chromium oxide layer of stainless steel and makes it more vunerable to corrosion RECOMM. to reduce corrosion -annealing after welding -using low carbon stainless steel (L) so that C does not precip. out |
|
|
Case Study Conclusions:
Natural Gas Pipeline Proposal |
-material mechanical properties to be considered: strength, hardness, toughness, and ductility
-chem properties: alloy content, corrosion resistance -phys properties: specific heat, coeff of thermal expansion |
|
|
How can alloying elements be used effectively to improve the hardenability of a substance?
|
the atoms of alloying elements can be distributed into a metal and increase the hardenability by increasing the carbon equivalent and giving the material and the hardenability of a high carbon content steel
|
|
|
What are the most important elements for hardenability?
|
sufficient carbon content
adequate quenching |
|
|
Why do sulfur and phosphorous improve machinability but reduce weldability?
|
high sulfur content causes cracks in welds
sulfur causes low strength in the solidifying metal |
|
|
What is the Jominy-End Quench Test?
|
- Sample is heated to austenitizing temp and one end is
Quenched in water. Bar cools at different rates depending on proximity to quench medium. Hardness is measured at prescribed distances and plotted - Used as measure of hardenability, to determine if through hardening will occur, |
|
|
What alloying elements are hardenability improvers?
|
Nickel, Manganese, Silicon, Boron, Moybdenum, and Vanadium
Ni, Mn, Si, Mo, V, B |
|
|
What alloying elements are machinability improvers?
|
Sulfur/Phosphorus and Lead
|
|
|
What alloying elements are corrosion resistance improvers?
|
Chromium and Copper
|
|
|
What alloying elements are toughness/strength improvers?
|
Nickel, Silicon, Chromium, Molybdenum, and Nitrogen
Cr & Mo (high temp strength), Ni, Si, N (acts like carbon) |
|
|
What are the through-hardening grades of alloy steel and their advantages?
|
>0.3% alloys will harden through heavy sections
has a 4x or 5x higher yield strength than a mild steel |
|
|
What are the carburizing grades of alloy steel and their advantages?
|
<0.2%
have better hardenability than low-carbon steels better mechanical core properties |
|
|
What is the designation and purpose of H steels?
|
H suffix (AISI 4140H=UNS H41400)
guaranteed hardening |
|
|
What is the designation and purpose of B steels?
|
Boron hardens in extremely small amounts (0.0005% - 0.003%)
which is cheaper for high production parts; AISI XXBXX |
|
|
What is the carbon content and purpose of High-Strength Low Alloy steels?
|
<0.2% C
- Higher mechanical properties, greater resistance to corrosion than traditional carbon steels - Intended for structural applications with welding |
|
|
What is the typical finishing of High-Strength Low Alloy steels?
|
- Hot-finished, not hardened, ferrite/pearlite microstructure,
solid-solution strengthened in ferrite matrix |
|
|
What is microalloying (wrt to high-strength low-alloy steels)?
|
additions of small amts of alloy for strengthening
HSLA steels usually have <0.5% of other elements |
|
|
What is the role of copper in high-strength low-alloy steels?
|
in small concentrations (<0.5%), produces solid solution strengthening of ferrite and improves the atmospheric corrosion resistance 4x
"weathering steels" have copper |
|
|
What is the composition and purpose of Austenitic-Mn steel?
|
(1-1.4% C, 10-14% Mn)
- These have the ability to work harden under impact -are commonly used in construction equipment -are weldable -Austenite is stable at room temp because Mn content |
|
|
What is the purpose of Mill-Treated Steels?
|
- Close to HSLA but other than hot-finished, welding issues
but suitable for structural applications, -high-strength structural fabrication, martensitic, special welding techniques required - Used for submersibles and pressure vessels |
|
|
What do the numbers correspond with in the following:
HY-80, HY-100 |
-80/100 stand for min yield strength (ksi)
|
|
|
What are ultra-high-strength steels?
|
Catchall category that includes alloy steels, tool steels, steels w high concentration of alloying elements, and stainless steels
have a higher yield strength with measured toughness |
|
|
What is Maraging steel 300?
|
- Good weldability, 300 ksi tensile strength
|
|
|
What is hot shortness?
|
FeS develops at grain boundaries with low melting temp
|
|
|
What are Free-Machining steels?
|
Forms smaller chips which means less energy, friction and heat and so less intensive during machining
|
|
|
How would any HSLA steel with a strength of 50 ksi appear on a construction drawing?
|
ASTM A 575, Grade 50
|
|
|
What is ASTM A538 Grade C commonly referred to?
|
Alloy steel class
UNS K93120 |
|
|
What does carburizing achieve?
|
Increases surface hardness
|
|
|
What does quenching achieve?
|
hardening in carburizing process
|
|
|
What does tempering achieve?
|
improves toughness
|
|
|
What are the primary differences between tool steels and basic carbon and alloy steels with respect to quality of the materials used, cost and manufacturing practices.
|
The manufacturing practices used to produce tools steels include special types of melting practices
that impart cleanliness and superior alloy control in the steels. The amount of alloy is usually greater than basic alloy steels and the cost is very expensive, to expensive for mass production. have much more rigid inspection and quality control standards. |
|
|
what is the most significant microconstituent that separates tool steels from carbon and alloy steels?
|
Alloy Carbide
Hardened structure of martensite w some volume fraction of alloy carbides (15-30%) The formation of alloy carbides replaces the ferrite matrix. |
|
|
How do tool steels differ from carbon and alloy steels?
|
- Tool steels possess the following characteristics as compared with carbon and alloy steels:
- Better hardenability and harden deeper - Better heat resistance or hardness at elevated temperatures - Easier to heat treat because of alloy content - Better resistance to abrasion - less prone to softening effects of heat |
|
|
What are the prefixes for cold work tool steels?
|
W (water hardening)
O (oil hardening) A (med alloy air hardening) D (high C, high Cr) |
|
|
What is the prefix for shock resisting tool steels?
|
S
|
|
|
What is the prefix for hot work tool steels?
|
H
|
|
|
What are the prefixes for high speed tool steels?
|
M
T |
|
|
What is the prefix for mold tool steels?
|
P
|
|
|
What is the prefix for special purpose tool steels?
|
L
|
|
|
What causes distortion in tool steels?
|
The type of quench or severity
avoided by adding more or different alloys to produce a material that can be air-hardened and avoid the distortion. |
|
|
What is passivity?
|
corrosion resistance from a surface condition that inhibits electrochemical action between a metal and its environment
when a metal is unattacked in an environment known to be capable of causing attack removing the passive film on a metal can lead to corrosion |
|
|
What are stainless steels wrt to chromium content and passivity?
|
>= 10.5% Cr content
the more chromium, the slower the corrosion rate passivity in an oxidizing environment (max usually 18% Cr) |
|
|
What major advantages do stainless steels have over carbon and alloy steels?
|
chromium concentration prevents corrosion
high carbon content and other alloys prevent passivity |
|
|
What is the designation for stainless steels wrt ASTM and UNS and the alphanumeric system used in the US?
|
The US alphanumeric system breaks down the steels based on use characteristics. For example,
M and T steels are High-Speed Tools. ASTM A681, A686, A600 and A597 cover most tool steels and should be noted on a tool drawing, especially if it will be manufactured OCONUS. Combined With a UNS number, the ASTM specification will identify particular tool steels. For example, ASTM A 681 type T72301 is W1 tool steel. UNS number always starts with a T for Tool steels. |
|
|
What is stress-corrosion cracking and why aren't ferritic stainless steels as susceptible to it as other stainless steels?
|
material deterioration due to cracking
spontaneous corrosion induced cracking of a material under static stress |
|
|
Explain 475C embrittlement
|
poor weldability results from the formation of embrittling phases and carbid precip. on cooling from welding temperatures
ferritics are subject to 475C embrittlement when ferritic stainless steel is subjected to use temperatures it decomposes into two separate BCC phases which has the effect of lowering ductility and impact strength ferritic stainless steels have BCC Microstructure, low carbon contents (<0.2% usually), Chromium ranges from 16-20% and are not subject to SCC |
|
|
Why do martensitic stainless steels undergo a crystal transformation when heated and quenched?
|
Martensitic stainless steels have 12-18%Cr, 1.2%C
the higher the C content further expands the gamma loop when heated, transforms to austenite, which makes quench hardening possible |
|
|
Describe how Gibbs Free Energy and the Cell Potential can tell us if corrosion will occur.
|
The cell potential can tell us if a reaction is possible (hence, there is a difference between the cathode and
anode. The magnitude or amount of energy can be used to determine the likelihood that a reaction will proceed. The driving force (E), measured in volts, will always be positive for spontaneous reactions. (note: when using an emf series to calculate cell potential, always correct the sign of the oxidation Reaction, to account for it not being a reduction.) |
|
|
Describe the concept of passivity and how the products of a corrosion cell can cause this to occur.
|
- Passivity is a reactive metals ability to become corrosion resistant. A passivating material such as
Titanium will form a layer (usually so thin it is not visible) of corrosion products that slows corrosion almost completely. The passive film is mostly composed of metal oxides and hydroxides. - Example: Stainless Steel with more than 18% Cr, form an oxide film (Cr2O3) |
|
|
how does fluid velocity affect the corrosion rate?
|
Fluid Velocity: Increased velocity may increase O2 levels (supply to cathode). In an active metal,
corrosion rates will increase. In passive metals, velocity will have limited effects once a thin film is formed. If the film becomes too thick, velocity will erode the film and corrosion will continue. Critical velocity, is a measure of the point at which this will occur (plain copper = 2-3 fps, admiralty brass = 5-6 fps) |
|
|
how does passivity affect the corrosion rate?
|
Passivity: Can drop corrosion rates by several orders of magnitudes.
|
|
|
how does polarization affect the corrosion rate?
|
Polarization: Potential difference between anode and cathode changes as corrosion continues to
Somewhere between the original anode and cathode potential. |
|
|
how does concentration of electrolytes affect the corrosion rate?
|
Concentration of Electrolyte: Can spur a reaction to occur such as in an oxygen concentration
cell or when pH differs throughout a solution. |
|
|
how does Metallurgical Differences in a Metal affect the corrosion rate?
|
Metal-ion concentration cells can develop, pitting
|
|
|
how does temperature affect the corrosion rate?
|
Increased temperatures increase reaction rates, diffusion rates
the rate of dissolution of gases in water. ionic conduction is increased in water and pH is lowered. Some metals demonstrate passivity at high temperatures and not low temperatures. |
|
|
how does stress affect the corrosion rate?
|
Stress: Many forms of stress-assisted corrosion. Coupled with corrosion, a material under stress may
fail catastrophically. |
|
|
List both ways to calculate cell potential from standard EMF tables.
|
1. Change anode sign:
Ecell = Ecat,red + Eanode, ox 2. Keep anode sign same: Ecell = Ecat,red - Eanode, ox |
|
|
How does the environment affect galvanic corrosion?
|
environment determines whether the metal acts as the anode or cathode
|
|
|
How does the anode/cathode area affect galvanic corrosion?
|
reaction takes place faster on anode side than cathode side
if cathode is BIG and anode is SMALL then corrosion takes place faster |
|
|
How does the distance affect galvanic corrosion?
|
the more distance, the harder it is for electrons to flow and corrosion to take place
|
|
|
How does a new metal and old metal together affect galvanic corrosion (same composition)?
|
the new metal may corrode faster since the old metal is more passive
|
|
|
What is dealloying corrosion?
|
slow progression but can be catastrophic wrt the strength of metal
internal to alloy (varying composition) results in potential difference dezinctification: Brass>13% Zn |
|
|
What is an oxygen-concentration cell?
|
Cathode has high oxygen
Anode has low oxygen O2 + 2H2O + 4e- --> 4OH- |
|
|
What is a pH concentration cell?
|
variation in pH (<4) can result in rxn
|
|
|
What is crevice corrosion?
|
corrosion occurring in crevices where there is a diff in 02 levels
crevices in metals where deposits form |
|
|
What is pitting?
|
local corrosion damage characterized by surface cavities after break-down of film or where stagnant solutions are
can perforrate thin wall rapidly growth in the direction of gravity pitting area is active |
|
|
What is microbial growth?
|
fungus/algae causing difference in O2 levels and resulting in corrosion
|
|
|
On what properties should selection criteria of alloy steels be based on?
|
- Selection criteria should be based on mechanical and
hardenability of alloy (if based on properties alone) |
|
|
What are some advantages to using aluminum vs steel?
|
more corrosion resistant
better heat/electrical conductor 1/3 weight of steel 1/3 stiffness of steel |
|
|
Why has aluminum only been used for the past 60 yrs as an engineering metal?
|
Because of the inertness of Al cmpds, it took 60 years of research to find an economically acceptable way to make Al from ore
|
|
|
What % of the earth's crust is Al?
|
8%
|
|
|
What is the crystalline structure of Al?
|
FCC
|
|
|
weight (density) of Al
|
low density
|
|
|
thermal/electrical conductivity of Al
|
higher conductivity than Cu per unit mass
|
|
|
resistance to corrosion of Al
|
good for applications involving atmospheric corrosion resistance
|
|
|
Is Al magnetic?
|
Al is non-magnetic
|
|
|
formability and machinability of Al
|
good formability and easily machined
|
|
|
weldability of Al
|
most Al alloys are weldable
|
|
|
5052-H
|
A95052
H: strain hardened |
|
|
6061-T6
|
A96061
T: thermally treated T6:furnace soln heat treated, quenched, furnace aged |
|
|
7075-O
|
A97075
O: annealed (wrought alloys only) |
|
|
What is a common Al extrusion product that could be considered important for Naval Architects?
|
sailboat masts
|
|
|
Explain how Al's limited solubility at room T with alloy contributes to it's ability to strengthen/harden
|
at room T: solubility <0.02% and precipitation hardening is possible
|
|
|
Potential use of Al (wrt to corrosion resistance) in salty air
|
Al has a extremely low corrosion rate in salty air
high corr. rate the 1st 2 years but afterwards, surface is covered with oxide corr. product (patina) and has a low corr. rate |
|
|
Potential use of Al (wrt to corrosion resistance) in seawater
|
5xxx series acceptable
prone to pitting requires cathodic protection/coatings stainless steel fasteners/attachments are good |
|
|
Potential use of Al (wrt to corrosion resistance) in water (piping)
|
not widely used in plumbing systems b/c H2O containing heavy metal ions
(Cu, Pb, Ni, Tin) leads to pitting |
|
|
How are pure Ni and Steel similar?
|
similar:
melting pt appearance modulus of elasticity density coeff of thermal expansion |
|
|
How are pure Ni and Steel different?
|
"Ni has 2x better thermal/electical conductor
magnetic permeability characteristics |
|
|
What are some uses of Ni?
|
(nickel-based super alloys) jet engine parts
high T electrical conductors and wire terminals magnetic shielding of electronic devices tape recording heads (nichrome) resistance heating elements |
|
|
What is the UNS # and primary alloys of Monel 400?
|
N04400
Ni, Cu |
|
|
What is the UNS # and primary alloys of Inconel 625?
|
N06625
Ni, Cr |
|
|
What is the UNS # and primary alloys of Incoloy 825?
|
N08825
Ni, Cr, Fe |
|
|
What are characteristics of Monel 400?
|
resistant to neutral waters, seawater, and some acids
resists errosion corrosion to high velocities strong passive film may cause severe galvanic attack of less noble materials when coupled suffers uniform corrosion, pitting, and crevice attack uses: valves, pumps, prop shafts, fixtures, fasteners, handling acids |
|
|
What are characteristics of Inconel 625?
|
high strength and toughness to 1800F (980C)
resists erosion corrosion to high velocities and highly resistant to most forms of attack uses: wire rope, propeller blades, fittings, springs, fasteners, parts where little/no corrosion can be accepted not susceptible to carbide precipitation during welding and subsequent intergranular attack |
|
|
What are characteristics of Incoloy 825?
|
resistant to chloride SCC suffers crevice corrosion and pitting
uses: desalination plant components and heat exchangers exceptionally resistant to sulfuric and phosphoric acids and to seawater |
|
|
How can Monel 400 be hardened or strengthened?
|
coldwork or precipitation hardened
|
|
|
How can Inconel 625?
|
solid solution strengthening
|
|
|
How can Incoloy 825?
|
solid solution strengthening
|
|
|
What is the difference between cupronickels and monels?
|
Cupronickels aren't subject to erosion up to certain velocities
Monels are not Cupronickels are primarily Cu Monels are primarily Ni Both are unlimited SS |
|
|
How is zinc used to protect steel from rusting?
|
zinc coats steel surfaces by hot-dip galvanizing, electrode position, diffusion coating, metal spraying, and zinc-rich paints
due to the low corrosion rate of zinc, a coating of 0.003 in may provide protection for as long as 30 years in non-coastal environments not corrosion resistant in seawater zinc is anodic to most metals so a scratch or machined area will still be protected by the nature of the zinc anodic coating the zinc will be corroded in preference to the exposed steel |
|
|
What are the most important physical properties of Titanium and why?
|
low density (1/2 the density of steel)
high modulus of elasticity very high specific strength high melting point better mechanical properties can be better than those of many alloy steels |
|
|
Why isn't Ti considered a refractory metal when it has a high melting point?
|
it is not usually used for high-temperature applications
|
|
|
How is Ti-6Al-4V solution treated to form an unstable but long-lived higher strength beta phase?
|
at room temperature, Ti is crystal structure alpha-phase titanium
at 1620F (882C) BCC beta-phase titanium if quenched above its transformation temperature, high-strength martensite is formed if both phases-->precipitation hardening |
|
|
How is commercially pure titanium strengthened?
|
it is strengthened with elements such as carbon, oxygen, nitrogen, and hydrogen going into interstitial solid solution in it
|
|
|
What is the UNS # and primary alloys of Unalloyed Ti?
|
R50250
Ti |
|
|
Why is Ti expensive (wrt corrosion resistance)?
|
excellent corrosion resistance to seawater and aqueous chloride solutions over a wide range of temperatures and concentrations
resistant to a wide range of oxidizing media such as nitric acid resistant to mildly reducing acids non-toxic and so it can be used to handle foods |
|
|
What properties make Ti a useful material?
|
strength
corrosion resistance high melting point |
|
|
What is the UNS # and primary alloys of Ti-6Al-4V?
|
R56400
Al,V,(<0.25% Fe)" |
|
|
Why is concrete an important engineering material?
|
hardens in water
inexpensive available durable, strong, versatile can be shaped |
|
|
What are the components of concrete?
|
Binding agent (cement)
Filler (rocks) Initiator (water) Reinforcement, admixtures (ex: rebar) |
|
|
5 basic types of cement
|
1. General Purpose
2. Modified General Purpose 3. High-Early-Strength 4. Low-Heat-of-Hydration 5. Sulfate-Resisting |
|
|
Heat of Hydration
|
heat caused by reaction with water
adiabatic system (>1.6ft in thickness) can get huge thermal stresses and tensile cracking |
|
|
Equation to estimate concrete strength based on water/cement ratio
|
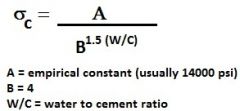
|
|
|
How does the amount and gradation of aggregates affect properties and cost of concrete?
|
It is harder to work with if there is more aggregate,
but becomes stronger (inexpensive) |
|
|
What is the OD and SSD state of aggregate?
|
OD- oven-dry, no water content
SSD- saturate surface dry SG= 2.0 to 3.0 |
|
|
4 basic types of admixtures
|
1. workability
2. set control 3. strength 4. durability |
|
|
3 basic methods of reinforcing concrete
|
1. pre-stressed
2. post-tensioned 3. welded-wire fabric |
|
|
What is sulfate attack and corrosion of reinforcing steel and what are their causes?
|
sulfates in soils/ground water- attack C3A and destroys strength of cement paste
|
|
|
What is a plastic and what is the basic organic substance is it produced from?
|
made from crude oil/natural gas
materal that contains an as essential ingredient an organic substance of large molecular weight solid in its finished state at some pt in its manufacturing/processing can be shaped by flow made up of hydrocarbons synthetic polymers |
|
|
What are the two basic types of plastics?
|
Thermoplastics- soften and melt when heated, hardens when cooled
Thermosets- doesn't melt, instead breaks down permanently when heated |
|
|
PET plastic
|
can have different densities
bends easily high impact strength |
|
|
Polyethylene
|
rigid
strong resistance to acid floats |
|
|
PVC
|
very rigid
|
|
|
Acrylic-Nylon
|
higher tensile strength (than Polypropylene)
low friction surface high melting pt |
|
|
Polypropylene
|
good tensile strength
good impact strength at low temps good stiffness |
|
|
How does production of plastic compare to that of steel?
|
plastics are formed into usable solid shapes by casting, sintering, or melt processing (like steels)
|
|
|
What is an addition polymerization reaction?
|
A + Initiator -> A-A-A
monomers linked |
|
|
What is a condensation polymerization reaction?
|
A + B -> C-C-C
water is commonly a by-product |
|
|
What is a cross-linking polymerization reaction?
|
A + B-B-B -> A - (B-B-B)
new macro molecule is formed |
|
|
How does copolymerization alter the properties of a polymer?
|
improves mechanical properties
|
|
|
How does blending alter the properties of a polymer?
|
retains properties of primary polymer
|
|
|
How does alloying alter the properties of a polymer?
|
increases lubricity
reduces friction characteristics |
|
|
How does branching alter the properties of a polymer?
|
strengthens
stiffens |
|
|
How does cross-linking alter the properties of a polymer?
|
strengthens
increases rigidity lessens solubility lessens response to remelting |
|
|
How does chain-stiffening alter the properties of a polymer?
|
strengthens
stiffens |
|
|
How does chain-stiffening create a polymer that demonstrates stereospecificity?
|
it positions the pendent groups along the polymer chain in a regular fashion so that it is more stereospecific
|
|
|
How does stereospecificity and pendent group size determines if a polymer will be amorphous or semi-crystalline?
|
the higher the stereospecificity (or order) of the bonds, the more crystalline the structure
|
|
|
Amorphous polymers
|
thermoplastics with extensive chain-branching, large pendent groups, and low stereospecificity
degrade near and above high temperatures thermosetting polymers with cross-linking can not fold up like accordion when cooling |
|
|
Semicrystalline Polymers
|
long, slender aliphatic chains and lower levels of chain-branching
amorphous phase has profound effects on polymer's mechanical properties high stereospecificity can fold up like accordion when cooled from liquid state usually do not have large pendent groups |
|
|
How can polymers' problem of degradation due to exposure to UV radiation be mitigated?
|
stabilizers and antioxidants (additives)
|
|
|
Does aluminum demonstrate unlimited solid solubility with some alloying elements?
|
No, aluminum does not demonstrate unlimited solid solubility with some alloying elements
|
|
|
How does Mg effect 5052?
|
improves strength
|
|
|
How does Mg/Si affect 6061?
|
improves strength formability
|
|
|
How does Zn affect 7075?
|
in combination with other alloys, promotes very high strength
|
|
|
What needs to occur in order for an alloy like aluminum to demonstrate enough solubility for precipitation hardening to occur?
|
Raise the temperature
|
|
|
Cupronickels and Monels have differing compositions of copper and nickel. A Monel has increased nickel which improves ___.
|
Ability to resist erosion corrosion
|
|
|
What kind of corrosion resistance does Ti demonstrate in seawater applications?
|
Ti demonstrates excellent resistance to corrosion in seawater applications
|

