![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
Effective nuclear charge trend
|
Increases L --> R
Not much change T --> B |
|
|
Covalent radius trend
|
Decreases L --> R
Increases T --> B |
|
|
Ionisation energy trend
|
Increases L --> R
Decreases T --> B |
|
|
Electronegativity trend
|
Increases L --> R
Decreases T --> B |
|
|
Inert pair effect
|
P-block elements often form compounds in which they are in an oxidation state 2 less than the group oxidation state. Increasing tendency for the s2 pair not to be used in bonding as you go down the group. Bond energies also fall - not enough to compensate for promoting an s electron to make it available for bonding.
|
|
|
How does difference in electronegativity affect a bond strength?
|
A greater difference in electronegativity between two elements will lead to a stronger bond between them
|
|
|
How do multiple bonds change going down a group?
|
Multiple bonds decrease in strength going down a group due to weakening pi bonds and poorer overlap
|
|
|
Effect of size on coordination number
|
Larger atoms can support higher coordination numbers
|
|
|
Trends between groups 14-17
|
One extra electron per atom going from 14 - 15, 15 - 16 etc.
Group 14 - 4 bonds Group 15 - 3 bonds, 1 l.p. Group 16 - 2 bonds, 2 l.p's Group 17 - 1 bond, 3 l.p's |
|
|
How to rationalise compounds (4 points)
|
i) Change in electronegativity
ii) Pi-bonding iii) Element size iv) Vacant orbitals |
|
|
Electronegative E - Is E-OH acidic or basic?
|
E-OH --> EO- + H+
Acidic |
|
|
Electropositive E - Is E-OH acidic or basic?
|
E-OH --> E+ + OH-
Basic |
|
|
VSEPR
|

|
|
|
Wade's Rules
|
1) Count all valence electrons
2) Divide by 2 to get no. of pairs 3) Subtract 1 pair for each exohedral B-H 4) Remaining pairs are PSEP's 5) N+1 = Closo N+2 = Nido N+3 = Arachno (n = number of vertices) |
|
|
Anomalies in periodicity
|
Usually reflect presence of d or f rows which have poor shielding
|
|
|
Borane reaction with base
|
[BnHn]2-
|
|
|
Boranes reaction with alkynes
|
Carboranes e.g. C2B10H12
|
|
|
Properties of silicones
|
Thermal oxidative stability
Flexible due to low energy bending of bonds Hydrophobic Me groups can be substituted |
|
|
Formation of phosphazines
|
[NH4]Cl + PCl5 + heat
|
|
|
Bonding in phosphazines
|
Electrons donated from N lone pair to vacant sigma* orbital on P
|
|
|
Reaction of phosphazines with heat then nucleophile
|
Polymer with Cl groups substituted for Nu
|
|
|
Formation of S4N4 (and S8)
|
[NH4]Cl + S2Cl2 + heat
|
|
|
Reaction if S4N4 with heat and Ag
|
S2N2 and (S=N) polymer
|
|
|
Properties of (S=N) polymer
|
Metallic conductor
Low temperature superconductor |
|
|
Formation of ammonia borane
|
MeNH2 + BH3
|
|
|
Effect if oxidation state on electronegativity
|
Higher oxidation state --> more electronegative
|
|
|
Heating ammonia borane
|
100C (-H2) - cyclic boron nitride
200C (-H2) - n methyl borazine (cf benzene) Further heating - hexagonal boron nitride (cf graphite) Very high temp and pressure - cubic boron nitride (cf diamond) |
|
|
Formation of phosphazines
|
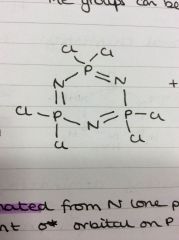
[NH4]Cl + PCl5 + heat
|
|
|
3 centre 2 electron bond
|
3 atoms share 2 electrons
E.g. B-H-B - one electron from each boron shared over the three atoms including H+ |
|
|
Why do 4p elements experience a greater effective nuclear charge?
|
Presence of 3d electrons - poor shielding. So electrons are held more tightly than would be expected.
|
|
|
Homonuclear bond energies
|
Weak between electronegative atoms due to lone pair repulsion. Decrease down a group as orbitals get bigger and overlap becomes poorer.
|
|
|
Heteronuclear bond energies
|
2A-B > A-A + B-B
Change in electronegativity strengthens the bond |
|
|
Amphoterism
|
Can act as both acidic and basic
|
|
|
Structure of dihydrates
|
Larger atoms can support a larger CN and disfavour double bonds - so octahedral with (OH)6 is preferred.
Smaller atoms prefer a H bonded adduct with double bonds to oxygen. |
|
|
Hydrolysis of xenon compounds
|
Leads to oxides, oxoanions and oxyfluorides
|
|
|
Affect of oxidation state on acidity
|
Higher oxidation state - more acidic
|
|
|
Oxidation of Xe2 to [Xe2]+
|
Makes it more stable as bond order increases from 0 to 0.5. Electrons removed from antibonding orbital.
|

