![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
What are the glycolytic enzymes that catalyze the irreversible steps in glycolysis.
|
1) Hexokinase (glucokinase in liver)
2) PFK -1 3) Pyruvate Kinase |
|
|
What are the enzymes that catalyze the irreversible steps in gluconeogenesis?
|
1) Glucose 6-phosphatase
2) Fructose 1,6 Bisphosphatase 3) Pyruvate carboxylase 4) PEP carboxykinase |
|
|
What is the function of Hexokianse, where is it found and what inhibits it?
|
Hexokinase is found in most cells and traps glucose when phosphorylating it to Glucose 6-P.
Trapping prevents transport out of the cell (RBC) via GLUT-1 transporters. When glucose 6-P accumulates it inhibits hexokinase from making more. Hexokinase is not needed for glycolysis during glycogen degradation (ATP is saved) |
|
|
What is the function of glucokinase, where is it found?
|
Glucokinase is found in hepatocytes and B-cells in pancreas.
-It reduces high blood glucose levels by taking it in via GLUT-2. Genetic deficieny can lead to maturity of onset diabetes of young type 2 (MODY 2) In B-cells, glucokinase is involved iwht recognition of high glucose to release insulin. |
|
|
How is glucokinase inhibited?
|
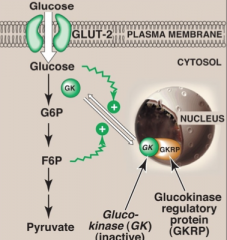
It is not product inhibited.
In the presence of high fructose-6-phosphate, glucokinase translocates and binds tightly to GKRP (glucokinase regulatory protein) in the nucleus, making it inactive. When glucose levels are high in blood and hepatocytes (GLUT-2), glucokinase is released from GKRP. |
|
|
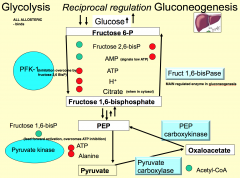
How is PFK-1 in general regulated?
|
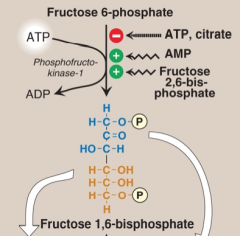
Glycolysis results in ATP generated. PFK-1 is inhibited by physiological ATP levels (when the objective to generate ATP is achieved).
It is allosterically inhibited by ATP and citrate. Citrate arises from FA synthesis in liver. And high H+ concentration. It is allosterically activated by AMP (signals low ATP - skeletal muscle, never in liver (always high ATP) and by Fructose 2,6 BP (made by PFK-2 - inhibits fructose 1,6 bisphosphatase, overcomes high ATP inhibition in liver). |
|
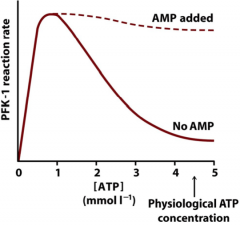
Explain this graph?
|
At low ATP levels, PFK-1 is very active - resulting in ATP generation from glycolysis. ATP production eventually leads to decrease PFK-1 activity. Addition of AMP (common in muscles) can overcome ATP inhibition.
|
|
|
What is the purpose of glycolysis in the liver?
|
It's function is not to form ATP, because of high ATP already in the liver that would inhibit PFK-1.
Rather its function is to reduce high blood glucose levels after a meal. A high insulin/glucagon leads to dephosphorylated bifunctional enzymes that activates PFK-2 to make fructose 2,6-BP, also makes protein phosphatase which activate phosphorylated pyruvate kinase (was phosphorylated by protein kinase A) |
|
|
How is it possible to perform glycolysis
at physiological ATP concentrations in the liver ?  |
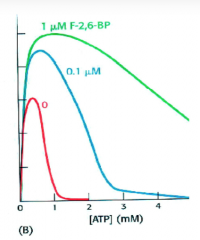
ADDITION of FRUCTOSE 2,6 BP
PFK-1 inhibition by high ATP in the liver is overcome by production of fructose 2,6 bisphosphate by activation of PFK-2. PFK-2 is active in presence of high insulin/glucagon levels resulting in dephosphorylated bifunctional enzyme. |
|
|
What is allosteric regulation of pyruvate kinase during glycolysis? How does it differ in muscle, heart and brain? Liver?
|
Once PFK-1 forms fructose 1,6-BisP it is committed and is a feed forward allosteric activator of pyruvate kinase.
Pyruvate kinase is feed-back inhibited by the generation of ATP, this is overcome by accumulation of fructose 1,6 BisP In muscle, heart and brain, they are not allosterically activated, but instead PK isozymes already have high affinity for PEP. In liver PK is inhibited by alanine during active alanine-glucose cycle? |
|
|
What are the general allosteric regulations of glycolysis?
|
- ATP inhibits PFK-1 and PK
- AMP and fructose 2,6-bisP activate PFK-1 - Fructose 1,6-bisP activates pyruvate kinase in liver, RBC and proliferating cells |
|
|
KNOW THIS
|
|
|
|
What are the covalent regulations of gluconeogenesis and glycolysis?
|
Insulin and glucoagon.
Insulin dephosphorylates bifunctional enzyme to activate PFK-2 to form fructo 2,6 BisP. This inhibits 1,6-bisphosphatase and activates PFK-1 -- leading to glycolysis. Glucagon phoshporylates bifunctional enzyme, which degrades fructose 2,6 bisphosphate by activating fructose 1,6 BisPhosphatase. Leads to inhibition of PFK-1 by ATP. Glucagon also phosphorylates PK to inactive form. |
|
|
Is the degradation of TAGs in adipocytes connected to gluconeogenesis ?
|
Yes, low insulin/glucahon ration and epinephrine lead to degradation of TAGs in fat cells. Glycerol and FFA are released into blood and transported to the liver and glycerol is used directly for gluconeogenesis (via DHAP_.
FA are degraded to acetyl-CoA which activates Pyruvate carboxylase. |
|
|
What is the affect of ethanol consumption on the liver?
|
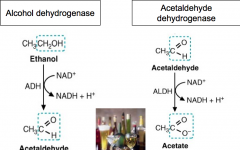
It leads to abnormally high NADH/NAD+ ratio in cytosol and in mitochondria.
Alcohol dehydrogenase converts ethanol to acetaldehyde which generates NADH in the cytosol. Acetalaldehyde is dangerous and can cause damage to the liver. To prevent is, Acetaldyhyde dehydrgenous is form to produce acetate + NADH -which leaves the blood. |
|
|
What is the affect of high NADH levels on gluconeogensis?
|
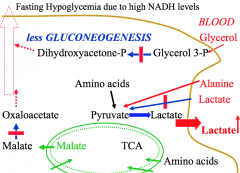
Run out of precursors.
-Lactate cannot be used to form pyruvate and stays in blood. -Glycerol 3-P cannot be used to form DHAP. -Glucogenic amino acids cannot be used as efficiently as oxaloacetate cannot be formed from malate in cytosol This decrease gluconeogenesis and can lead to fasting hypoglycemia. |

