![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
What are autocoids?
|
Autocoids – are chemical mediators that are synthesized and function in a localized tissue or area and participate in physiologic or pathophysiologic responses to injury
-they act only locally and therefore also termed “local hormone” -typically, autocoids are short-lived and rapidly degraded |
|
|
What are the classes of autocoids?
|
-can be divided into 3 categories based on chemical structure
1. Biogenic amines: Histamine, serotonin (5-hydroxytryptamine) 2. Polypeptides: Bradykinin, angiotensin 3. Lipid derived autocoids a) Eicosanoids – prostaglandings, leukotrienes, thromboxane b) Platelet activating factor |
|
|
Where is histamine come from?
|
1) Food & vagal stimulation can release histamine from the stomach mucosal enterochromaffin-like (ECL) cells. The release histamine then initiates gastric acid secretion
2) Allergic responses in the skin and lungs are due in part to histamine release from mast cells -mast cells are the primary cells that store histamine where it exists in a complex with heparin sulfate and chondroitin sulfate E in storage granules. The rate of histamine synthesis and turnover in mast cells is low -histamine is also found in CNS where it may act as neurotransmitter -many venoms and insect stings contain histamine, as well as other biologically active substances |
|
|
What kind of receptor is the H1 receptor?
|
H1 receptors are coupled to Gq protein-phospholipase C and mediate the following effects:
-contraction of smooth muscle and neuronal actions are due to increases in [Ca2+]i and activation of protein kinase C -relaxation of vascular smooth muscle involves Ca2+-induced formation of nitric oxide (NO) -mediate contraction of bronchiolar and intestinal smooth muscle, vasodilation of small arteries & veins, increased capillary permeability & puriritis *note relaxes bronchial smooth muscle in cats |
|
|
What kind of receptor is the H2 receptor?
|
B) H2 receptors are coupled to Gs protein-adenyly cyclase
-stimulation of these receptors activates adenyly cyclase and increases tissue cAMP levels -this is the method by which vascular smooth muscle relaxes and gastric acid secretion is stimulated |
|
|
What kind of receptor is the H3 receptor?
|
C) H3 receptors are coupled to Gi/o protein. Inhibition of the release of histamine and other neurotransmitters involves inhibition of cAMP synthesis, opening of K+ channels to increase K+ efflux, and closure of Ca2+ channels to block Ca2+ entry into the nerve
-located presynaptically on neurons and inhibit neurotransmitter release |
|
|
What are the effects of histamine on the cardiovascular system?
|
CARDIOVASCULAR SYSTEM:
-histamine dilates arterioles, capillaries, & venules, increases cardiac contractility & HR by activating both H1 & H2 receptors -there is a decrease in peripheral resistance (vasodilation), resulting in hypotension -the stimulation of cardiac activity involves a direct action and reflex activation of the sympathetic nervous system, which is activated by the low blood pressure -there is an increase in capillary permeability brought about by contracting endothelial cells, which exposes basement membrane = edema |
|
|
What are the effects of histamine on the respiratory system?
|
RESPIRATORY SYSTEM
-respiratory smooth muscle is contracted in most species via H1 receptors -there is also stimulation of glandular secretion and prostaglandin formation |
|
|
What are the effects of histamine on the glandular tissue?
|
GLANDULAR TISSUE
-sight & smell of food activate the vagus via the CNS to release Ach on parietal cells (M3-muscarinic receptor) and on enterochromaffin-like (ECL) cells (M1-muscarinic receptor) -the presence of food and an increase in antral pH initiate the release of gastrin which acts on CCK2-receptors of the parietal and ECL cells. Histamine is released from ECL cells that are close by, and activate parietal H2-receptors -Ach has both direct and indirect action on gastric acid production. Activation of M3-receptors directly activates the parietal cell, whereas activation of the M1-receptor on ECL cells releases histamine which in turn activates H2 receptors on the parietal cell -gastrin has both a direct and indirect action. Gastrin directly activates CCK2 receptors on parietal cells to increase gastric acid secretion and indirectly increases gastric acid secretion by activating the release of histamine from ECL cells, which again in turn activates H2-receptors -THUS HISTAMINE release is a major factor in the stimulation of acid production by both Ach & Gastrin |
|
|
What are antihistamines used for?
What is the difference between the newer & the older antihistamines? |
-antagonists that block H1-receptors are used in the treatment of allergic conditions such as hay fever, urticaria, drug sensitivity rashes, pruritis, & insect bites and stings
-they are not effective in asthma -older antihistamine drugs (e.g. chlorphenamine, alimemazine, prothazine) have anti-muscarinic actions and pass the blood brain barrier, commonly causing drowsiness and psychomotor impairment -newer agents (e.g. loratidine, ceirizine, fexofenadie) do not have atropine-like actions, because they do not cross the blood brain barrier to any extent, they cause much less drowsiness |
|
|
What are the eicosanoids? How are they made?
|
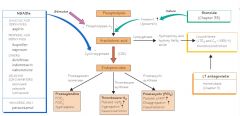
-the eicosanoids include the prostaglandins (PGs), thromboxanes (TXs), and leukotrienes (LTs)
-they are derived from polyunsaturated acids and arachidonic acid, a 20-carbon essential fatty acid with 4 double bones (20:4) as the primary substrate -arachidonic acid is released from membrane phospholipids primarily by phospholipase A2 in response to physical, chemical, hormonal, and neurotransmitter stimuli -arachidonic acid can be metabolized by 3 pathways: 1) the cyclooxygenase pathway leads to prostaglandin, thromboxane, and prostacyclin production 2) the 5-lipoxygenase pathway leads to synthesis of leukotrienes 3) the 15-lipoxygenase pathway |
|
|
Are eicosanoids preformed?
|
-in contrast to other autocoids, eicosanoids are not found preformed in tissues or cells
-they are formed de novo from cell membrane phospholipid, and have very short half lives – seconds to minutes |
|
|
What are eicosanoids important mediators of?
|
-important mediators of the inflammatory reaction
|
|
|
What type of receptors do eicosanoids act on?
|
-all eicosanoid receptors are G protein-coupled receptors
|
|
|
What are the forms of cyclooxygenase? Are they expressed all the time?
|
-cyclooxygenase exists in the body in 2 forms – COX1 that is present in most cells and is expressed all the time (constitutive COX) and COX2 that is induced in response to inflammatory states (inducible COX)
|
|
|
What does Prostacyclin PGI2 do?
|
o predominantly from vascular endothelium
o vasodilation o inhibition of platelet aggregation |
|
|
What does PGD2 do?
|
o predominantly from mast cells
o vasodilation o inhibition of platelet aggregation |
|
|
What does thromboxane do?
|
o predominantly from platelets
o vasoconstriction o platelet aggregation |
|
|
What is the action of PGE or EP1, 2 & 3 receptors? What else does it do?
|
o EP1 receptors – contraction of bronchial and GIT smooth muscle
o EP2 receptors – relaxation of bronchial, vascular and GIT smooth muscle o EP3 receptors – inhibition of gastric acid release, increased gastric mucus secretion (i.e. cytoprotective) -contraction of pregnant uterus and GIT smooth muscle -mediator of fever |
|
|
What is the action of PGF2α?
|
o acts on FP receptors in smooth muscle and corpus luteum
o produced in the uterus of non pregnant mares, cows, sow -PGF2α is the lutelytic hormone produced by the ovaries and endometrium, which causes ovulation and regression of corpus luteum (luteolysis) in cycling animals -it initiates luteolysis, which decrease plasma progesterone levels -it is also a stimulant of myometrium and is involved in parturition |
|
|
What are dinoprost “lutalyse” & cloprostenol “estrumate” used for? What are the side effects?
|
-PGF2α products
-used to decrease estrous length and therefore synchronize oestruse in mares & cows -used to induce parturition in cows – interval between induction and calving approx 24 hours. Also used in mares & sows -also an abortifacient in humans so should be used with extreme care by woman of child bearing age Side effects: acute bronchoconstriction in asthmatics, or other bronchial disease |
|
|
What is PGF2α used for in ophthalmology?
|
-PGF2α is also used in ophthalmic preparations in treatment of glaucoma (e.g. latanoprost)
|
|
|
What is an example of a prostraglandin we use in the gastrointestinal system?
|
-Misoprostol, a stable analogue of PGE1 is also used for its cytoprotective effects in gastric ulceration
-increases mucosal blood flow & mucus production, decreases gastric acid production |

