![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
34 Cards in this Set
- Front
- Back
|
What are enzymes? |
• Biological catalysts which speed up the rate ofa reaction, without altering the final equilibriumbetween reactants and products. • Extremely efficient, e.g. the enzyme catalasecatalyses the breakdown of its substrate H2 O2to water at a rate 1014 times faster than theuncatalysed reaction at 30C. |
|
|
What is the Effect of enzyme on activationenergy of a reaction? |
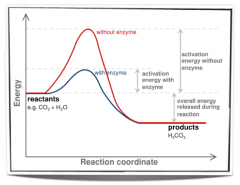
|
|
|
What is the Effect of an enzyme on a biologicalreaction? |
Increases the reaction velocity Lower Km value |
|
|
What are enzymes responsible for? |
Enzymes are responsible for the controlled“combustion” (oxidation) of foodstuffs |
|
|
What does enzyme specificity mean? |
Enzymes will usually catalyse only one type of reaction (e.g.alcohol dehydrogenase liver will oxidise primary alcohols toaldehydes) and will act on only a few related molecules(‘group specificity’ e.g. the above enzyme will oxidisemethanol, ethanol and propanol). |
|
|
Will some enzymes only act on one substrate? |
A few enzymes are so specific they will act on one substrate |
|
|
Can enzymes distinguish between isomers? |
If a natural compound can exist in two stereoisomer forms e.g.D-glucose and L-glucose, the enzyme concerned with itsmetabolism in the cell will usually act only on one isomer. |
|
|
What is specificity determined by? |
Specificity is determined by the presence of a groove or cleftof defined shape called the ‘active site’ into which only thesubstrate of the correct shape and charge can fit |
|
|
What can be the consequence of enzyme specificity? |
A group of enzymes present together in one compartment of acell, e.g. the cytoplasm of muscle cells, can give rise to acomplex and co-ordinated metabolic pathway in which the initialsubstrate D-glucose is converted through a sequence ofspecific enzyme catalysed reactions to the product lactic acid |
|
|
How are enzymes classified? |
Specificity of enzymes has led to a systematic classificationscheme, established by the I.U.B. Commission on Enzymes |
|
|
What are the 6 main classes enzymes are divided into? |
Enzymes are divided into 6 main classes according to thetype of reaction they catalyse e.g. Class 1, oxidoreductases,contains the enzymes catalase and alcohol dehydrogenase. |
|
|
How are the six classes then further divided? |
Six classes are then further divided into subgroupsaccording to their substrate or source.• Each enzyme is identified by its own individual 4 digitnumber (e.g. catalase is E.C. 1.11.1.6.) |
|
|
What do oxidoreductases do? |
Oxidoreductases catalyze the transfer ofhydrogen atoms and electrons Example - Lactate Dehydrogenase |
|
|
What do transferases do? |
Transferases catalyze the transfer of functionalgroups from donors to acceptors Example - Alanine aminotransferase |
|
|
What do lyases catalyse? |
Lyases catalyze the cleavage of C-C, C-O, or C-Nbonds (addition of groups to double bonds or formation of doublebonds by removal of groups) Example - ATP-citrate lyase |
|
|
What do hydrolases do? |
Hydrolases catalyze the cleavage of bonds bythe addition of water (hydrolysis) Example - Trypsin |
|
|
What do isomerases do? |
Isomerases catalyze the transfer of functionalgroups within the same molecule Example - Phosphoglucose isomerase |
|
|
What do Ligases do? |
Ligases use ATP to catalyze the formationof new covalent bonds19 • Example - DNA ligase |
|
|
What type of compound are enzymes? |
Enzymes are proteins i.e. they are composed of one ormore polypeptide chains folded into a complex 3-dimensional shape |
|
|
What is enzyme structure stabilised by? |
Enzyme structure is stabilised by many weak bonds e.g.H-bonds, electrostatic salt links, hydrophobic interactions |
|
|
Are these bonds easily broken? |
These weak bonds are easily broken e.g. by heating theprotein, giving rise to a disorganised or tangled structure inwhich the enzyme no longer has any catalytic activity(inactive, ‘denatured’ state). |
|
|
What does this property mean for the enzymes? |
This property makes enzymes very sensitive to changes intheir environment |
|
|
What does the active site contain to stabilise the transition state of the reaction? |
The active site of the enzyme contains functional groupsthat stabilize the transition state of the reaction |
|
|
What is the “Lock and Key” model for enzyme catalysis? |

|
|
|
What is the induced fit model of enzyme catalysis? |
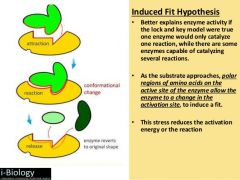
|
|
|
What is the Effect of temperature on enzymereactions? |
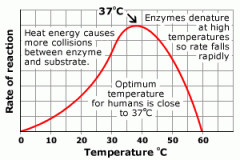
|
|
|
What is the effect of pH on enzyme activity? |
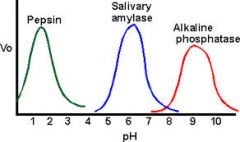
|
|
|
What are some inorganic elements that act ascofactors for enzymes? |
• Cu2+ — Cytochrome oxidase • Fe2+/3+— Cytochrome oxidase, catalase, peroxidase • K+ — Pyruvate kinase • Mg2+ Hexokinase, G-6-phosphatase, pyruvate kinase • Ni2+ — Urease • Se — Glutathione peroxidase • Zn2+ — Carbonic anhydrase, alcohol dehydrogenase,carboxypeptidase |
|
|
What are some iron containing enzymes? |
Iron-sulphur centre e.g flavin enzymes Heme group e.g cytochrome oxidase |
|
|
What are some co-enzymes? |
CH3 CH2 OH + NAD+ ----> CH3 CHO + NADH + H+ alcohol dehydrogenase succinate + FAD ----> fumarate + FADH2 succinate dehydrogenase glucose + ATP ----> glucose-6-phosphate + ADP glucokinase |
|
|
What are isoenzymes? |
Describes enzymes with different protein structureswhich catalyse the same reaction. |
|
|
What are they coded for by? |
Coded for by different genes |
|
|
Where are different isoenzymes found? |
Different isoenzymes often found in differentcellular compartments (cytoplasm or mitochondria),or different amounts in different tissues |
|
|
What are some examples? |
•Distinct biochemical roles •e.g. hexokinase /glucokinase. Lactatedehydrogenase |

