![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
What is the Chemical composition of a bacterial cell? |
• A cell maycontain c.1000different typesof small organicmolecule. • But many ofthese can beclassified into 4families: Sugars, amino acids, fatty acids and nucleotides |
|
|
What do the 4 families form? |
Four main families of small organic molecules formmomomeric building blocks for the formation of biologicalmacromolecules |
|
|
What functions do the monomers also have? |
Monomers may also have distinct functions: • Sugars and fatty acids: energy source • Nucleotide (ATP): energy carrier |
|
|
What are the features of carbohydrates? |
• Monosaccharides (simplesugars) are the building blocks ofcarbohydrates • Molecular formula oftenn x C H2O – n = 3, 4 ,5 or 6– e.g. C6H12O6 • Functional groups: carbonyl andhydroxyl • Hydrophilic, polar, water soluble |
|
|
How are monosaccharides classified? |
Monosaccharides are classed as aldoses or ketoses,depending on whether they contain an aldehyde ora ketone group. |
|
|
How does ring formation occur in carbohydrates? |
• in aqueous solution, 5 and 6 carbon sugarsspontaneously form ring structures • the carbonyl (aldehyde or keto) group reacts with ahydroxyl group |
|
|
What isomers do monosaccharides form? |
• Monosaccharides can occur as optical isomers or enantiomers (DorL-isomers) – mirror image forms • Most naturally occurring sugars are D-isomers • D-glucose (dextrose), but not L-glucose, can be metabolised by cellsin the glycolysis pathway |
|
|
What are the other isomers of glucose? |
Other isomers of glucose differ in the relative spatialarrangement of the carbonyl and hydroxyl groups, andare given different names: Epimers: stereoisomersthat differ in configurationat a single asymmetriccarbon Structural isomer |
|
|
What are the two isomers of glucose in ring formation? |
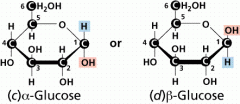
When glucose is in the ring structure, the hydroxylattached to carbon 1 (the aldehyde carbon) has twopossible positions (α- and β-). • The α- and β-forms interconvert rapidly in solution |
|
|
what is C1 in ring structure termed? |
C1 in the ringstructuretermed theanomericcarbon. |
|
|
What are anomers? |
α and β-D-glucoseare anomers(stereoisomers thatdiffer in configurationof the anomericcarbon) |
|
|
How are complex carbohydrates formed? |
Complex carbohydrates are formed by glycosidic bondsbetween monosaccharides |
|
|
What happens to the a or B configuration when the bond is formed? |
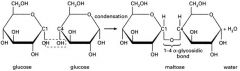
The α- or β-configuration is “locked” when the bond isformed α 1→4 linkage β 1→4 linkage |
|
|
What are disaccharides? |
two monosaccharides linkedby a glycosidic bond |
|
|
What are Polysaccharides: “many” saccharides? |
Polymers of glucose act as energy stores – Starch (amylose and amylopectin) in plants – Glycogen in animals |
|
|
What is the structure of glycogen similar to? |
Glycogen has asimilar chemicalstructure toamylopectin, butwith morebranches |
|
|
What can complex oligosaccharides form? |
Complex oligosaccharides (a “few” saccharides) canform recognition molecules on cell surfaces e.g. bloodgroup determinants |
|
|
How can sugars be changed? |
Sugars can be modified, and linked to lipidsor proteins |
|
|
What are lipids and fats? |
Lipids: molecules in cells that are waterinsoluble(hydrophobic) but soluble inorganic solvents – Triacylglycerols (fats and oils) – Glycerophospholipids and other membranelipids – Steroids and cholesterol |
|
|
What are fatty acids? |
Fatty acids are the monomeric building blocks oftriacylglycerols and glycerophospholipids |
|
|
What does the length and structural formula of the fatty acid carbon chain determine? |
The length and structural formula (saturated orunsaturated) of the fatty acid carbon chaindetermines its physical properties (shape, meltingpoint) |
|
|
How are triacylglycerols fomed? |
Triacylglycerols (triglyceride fats) are formed byester linkages between fatty acids and glycerol |
|
|
What are triacylglycerols useful for? |
• Important energy storagemolecules • Hydrophobic (insoluble)so stored as fat dropletswithin cells |
|
|
what are Glycerophospholipids? |
• Also based onglycerol, but onefatty acid isreplaced by aphosphate group • The phosphategroup is also linkedto a hydrophilic“head group” |
|
|
What are the characteristics of glycerophospholipids? |
• Glycerophospholipids are amphipathic (have ahydrophilic “head” and hydrophobic “tails”) • This causes them to aggregate in an aqueousenvironment • The phospholipid bilayer forms the cell membrane |
|
|
What is the steroid template the basis for? |
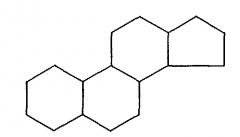
The steroid template (fused alkyl rings) is the basis forsteroid hormones and the sterol lipid, cholesterol. |
|
|
What is the role of cholesterol in cell membranes? |
• Cholesterol is a necessary component of animal cellmembranes • Rigid structure inserts between glycerophospholipids ––modulates membrane fluidity |
|
|
What is the role of nucleic acids? |
Ribonucleic acid and deoxyribonucleic acid (RNA andDNA), act as information molecules for the cell |
|
|
What do nucleotides consist of? |
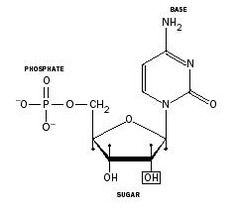
• Nucleotides, the building blocks of RNAand DNA, consist of: – a pentose sugar – a nitrogenous base – phosphate |
|
|
What does the base-pair sequence form? |
The base-pair sequence of DNA forms the genetic code |
|
|
What is another function of nucleotides? |
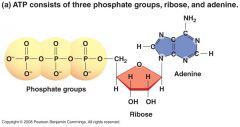
Nucleotides also have other functions, particularly ATP,which carries chemical energy in its phosphoanhydridebonds |
|
|
What is the main function of proteins? |
Proteins carry out the mechanical, structural andtransport functions of the body. |
|
|
What is their other crucial role? |
They also play a crucial role as enzymes (biologicalcatalysts). |
|
|
What varieties do proteins come in? |
Different proteins display a wide variety of shapes andsizes. |
|
|
What determines the structure of each protein? |
Each protein structure is unique and is determined bythe information in the DNA sequence of the gene. |
|
|
How do proteins fold up? |
Proteins fold up spontaneously from linear chainsof amino acids |
|
|
What are the building blocks of proteins? |
Amino acids are the building blocks of proteins |
|
|
How many amino acids are there? |
20 amino acids - common to all living organisms |
|
|
What are the characteristics of amino acids? |
Amino acids are water soluble and electricallycharged at physiological pH |
|
|
How does each amino acid differ? |
Each amino acid differs in the properties of itsside chain (R group) |
|
|
How are amino acids linked in proteins? |
In polypeptides (proteins), amino acids are linked bypeptide bonds |
|
|
What are not involved in peptide bonding? |
Side chains (R groups) are not involved in peptidebonding |
|
|
How are amino acids grouped? |
Amino acids can be grouped according tothe characteristics of their side chains. |
|
|
What are the Amino acids with side chains that are basic (positivelycharged) at physiological pH? |
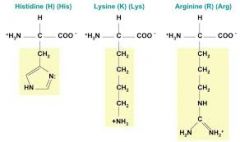
|
|
|
What determines protein folding? |
• Side chains of amino acids are not involved in peptidebonding • Interactions of side chains determine protein folding |
|
|
What are the amino acids that are acidic (negatively charged) at physiological pH? |
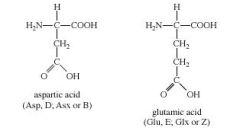
|
|
|
What are the non-polar, uncharged amino acids? |

|
|
|
What are the amino acids need to learn? |
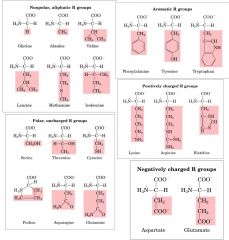
|

