![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What must happen for a reaction to occur? |
The reactant must collide |
It's a theory |
|
|
What are the two possibilities during a reaction? |
·Nothing Happens ·Reaction Happens |
|
|
|
What must be true for a reaction to occur? |
The Energy of Particles => Ea |
|
|
|
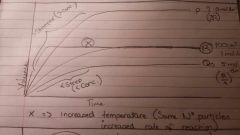
What happens to the gradient of the line when the concentration is increased? |
Increase (as does the volume) because there are more particles in a given volume |
|
|
|
What happens to gradient of the line when the temperature is increased? |
It increases |
The volume stays the same because there are the same number of particles in a given volume |
|
|
What happens to the gradient of the line when the concentration is decreased? |
It decreases as well as the volume because there are less particles in a given volume |
Decrease = Decrease |
|
|
What happens to the curve when the temperature is decreased? |
It moves up and to the left |

Maxwell-Boltzmann |
|
|
What happens to the curve when the temperature is increased? |
It moves down and to the right |
|
|
|
What does the highest point represent? |
The Most Probable Energy |
Not necessarily the average energy |
|
|
What is the activation energy? |
The Energy required for successful collisions to occur |
Can be represented by two letters |
|
|
What happens to the Activation Energy when a catalyst is present? |
It is lowered so that less energy is required by providing more pathways for the products |
Position |

