![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
111 Cards in this Set
- Front
- Back
|
A pH - pKa that results in a negative number means that [...] ? |
It means that there is an acidic environment. |
|
|
What impact does a high pKa have on the ionized state of an acid in a solution? |
The larger the value of pKa, the smaller the extent of dissociation at any given pH. |
|
|
Draw a curve with % nonionized form of a drug as a function of environment of the drug. Label the state of each acid/base in their particular environments. |
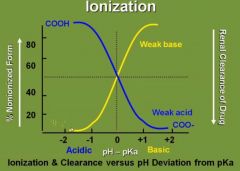
|
|
|
Draw the henderson-hasselbach equation. |
pH = pKa |
|
|
To increased renal clearance of a weak base drug you would ___________ the pH of the urine. |
Decrease. |
|
|
To increase renal clearance of a weak acid drug you would ___________ the pH of the urine. |
Increase. |
|
|
To absorb a weak acid drug you should keep it in what environment? |
Acidic |
|
|
To absorb a weak base drug you should keep it in what environment? |
Basic |
|
|
What is the pH of the stomach? |
1-2 |
|
|
What is the pH of the small intestine? |
6 |
|
|
What is the pH of the blood? |
7.4 |
|
|
What is the pH of the urine? |
4.5 - 8 |
|
|
Place a weak acid/weak base drug on a pH scale. Mark the different forms the drugs will appear as based on pH. |
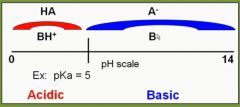
|
|
|
Where is a cephalosporin drug (with a hypothetical pKa of 5) more effectively absorbed (based on pH), in the stomach or small intestine? |
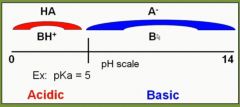
Stomach |
|
|
In what form is a drug renally cleared? |
Only free, unbound drug is filtered. |
|
|
What form of drugs are filtered in the kidney, non-ionized or ionized? |
Both. |
|
|
What form of a drug can undergo secretion and reabsorption in the kidney? |
Non-ionized form can be actively secreted and active or passive reabsorption. |
|
|
Mention 5 drugs or classes of drugs that are weak acids. |
(1) Aspirin |
|
|
Mention 4 drugs or classes of drugs that are weak bases. |
(1) Morphine |
|
|
When would you want to acidify and when would you want to alkalinize the urine to increase renal elimination of a drug? |
Acidification of urine --> Increased ionization of weak bases --> Increased renal elimination |
|
|
PCP is also known as ____________ (slang)? |
"Angel dust" |
|
|
PCP is an analogue of ______________. |
ketamine |
|
|
What happens after ingestion of PCP? |
Dissociative anesthesia (insensitivity to pain without loss of consciousness) and analgesia. |
|
|
How do you acidify the urine? |
(1) NH4Cl |
|
|
How could you alkalinize the urine? |
(1) NaHCO3 |
|
|
A man with end-stage liver disease becomes increasingly confused after eating a high protein meal. How could you ameliorate this situation? |
Lactulose, which is converted into lactic acid by gut bacteria. This low pH keeps ammonia ionized and not as readily absorbed (NH3 --> NH4+). |
|
|
What are the basic forms of drug transport? |
(1) Passive diffusion |
|
|
What basic mode of drug transport do drugs most often utilize? |
Passive diffusion |
|
|
Describe the energy requirement, requirement of carriers and saturability with each basic mode of drug transport. |
Passive diffusion - No energy required, No carrier needed, Not saturable |
|
|
Give an example of active transport of drugs. |
Tubular (proximal) secretion of penicillins and diuretics. |
|
|
What does pharmacokinetics and pharmacodynamics mean? |
Pharmacokinetics: What the body does to the drug. |
|
|
What does permeation mean? |
The ability of a drug to move within the body |
|
|
What is permeability dependent on? |
(1) Solubility |
|
|
Most drugs are [...] |
Weak acids or weak bases |
|
|
What is pKa? |
The pH at which weak bases and weak acids are present as 50% non-ionized and 50% ionized. |
|
|
In a nutshell, ionized drugs are ________ while non-ionized drugs are _______. |
water soluble; lipid soluble |
|
|
What is absorbed faster, IM or subcutaneous injection of a drug? |
IM = more vascular |
|
|
Draw a flow chart that encompass the term "Pharmacokinetics". |
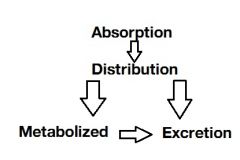
|
|
|
What is the bioavailability of IV drugs? |
100% |
|
|
What is the fastest route of absorption: |
B. Inhalation |
|
|
What does bioavailability mean? |
How much of the administered drug dose gets into the blood |
|
|
Draw a graph depicting the plasma drug concentration as a function of time when a drug is administered extravasculary. |
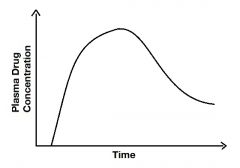
|
|
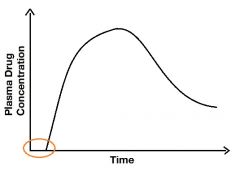
Describe the marked area. |
It's the lag period, it's the time from first administration until the time when molecules first appear in the blood. The flat line represents the drug in your alimentary tract before any molecules are present in your blood. |
|
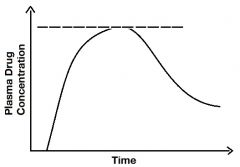
Describe the line. |
It's the Cmax of peak level of the drug. |
|
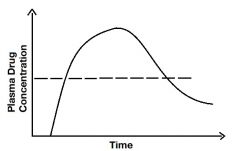
Describe the line. |
The line represents the plasma drug concentration (where it intersects with the graph) where you are first seeing a pharmacological effect of the drug. It's called the minimum effective concentration. |
|
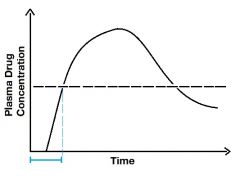
Describe the blue line. |
The blue line is the onset of activity. It includes the lag time (when it ascends). |
|
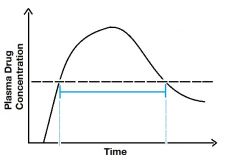
Describe the blue line- |
Duration of action. Notice how the graph is above MEC between the two points. |
|
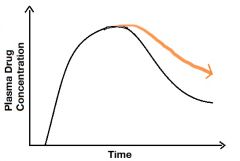
Describe the orange line. |
Decreased elimination of a drug. Could be a man with renal disease for example. |
|
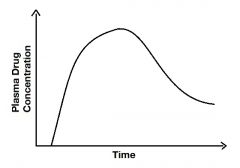
What does the area under this curve represent? |
Bioavailability |
|
|
How is bioavailability defined? |

f = fraction of the dose that is available in the blood |
|
|
How can we depict that the bioavailability of a drug is close to 100%? |
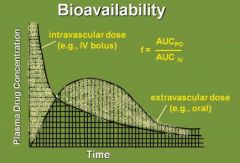
When the area in the shaded part of the curves are equal. |
|
|
What is one thing that greatly affects the bioavailability of a drug (f)? |
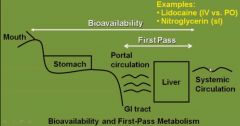
First pass effect |
|
|
What is the first pass effect? |
The rapid metabolism of a drug that may happen after it is absorbed into the portal circulation and reaches the liver. It may decrease the bioavailability of a drug. |
|
|
Why is IV lidocaine preferred over oral? |
Extensive hepatic first pass metabolism |
|
|
What does the pharmacokinetic term "distribution" include? |
Processes of distribution of a drug from systemic circulation into organs and tissues. |
|
|
Many drugs bind to what in the circulation? |
Plasma proteins. They remain in an equilibrium: |
|
|
Competition between drugs for plasma protein-binding sites may increase the _____________ , possibly enhancing the __________________. |
"free fraction"; effects of the drug displaced |
|
|
Sulfonamides are given to a neonate. The neonate develops kernicterus. What has happened? |
Sulfonamides are bound to albumin, displacing bilirubin. |
|
|
Warfarin is about ____% protein bound. |
98% |
|
|
What is the mechanism of action of sulfonamides? |
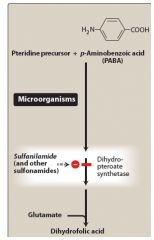
|
|
|
A man with a slowly progressing pneumonia is known to have a nocardia infection in the lungs. He is given sulfonamides. After a short time he starts to bleed from his gingiva and has easy bruising. What other drug was he taking at the time? |
Warfarin |
|
|
What drugs can penetrate the BBB? Give examples. |
Lipid soluble drugs of very low molecular weight. |
|
|
If a drug crosses the BBB, it will cross the __________. |
placenta |
|
|
What property would a drug have to have to be considered safe in pregnancy? |
(1) Water-solubility |
|
|
What would you choose in pregnancy, PTU or methimazole? |
PTU (PTU = P = P for Pregnancy) |
|
|
What anti-convulsant drug could you give to a pregnant woman with seizures? |
Phenobarbital (Phenobarbital = P = P for Pregnancy) |
|
|
What does volume of distribution mean? |
It's a pharmacokinetic parameter that correlates the dose of a drug with the plasma concentration: |
|
|
What happens to the Vd when a drug is highly plasma protein bound? |
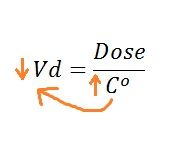
High plasma protein binding keeps more of the drug in the blood (increased Co) |
|
|
What happens to the Vd when a high percentage of a drug is sequestered in tissues? |
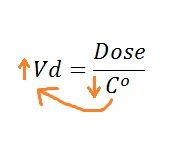
|
|
|
Digoxin is highly sequestered in tissues. Which drugs could displace it and enhance digoxin toxicity? |
Verapamil and quinidine. |
|
|
When considering total body water, intracellular fluid makes up how much? |
2/3 |
|
|
When considering total body water, extracellular fluid makes up how much? |
1/3 |
|
|
For an ideal 70kg body weight man, what normal values for the following: |
"60-40-20 rule": |
|
|
Plasma is what fraction of extracellular fluid? |
1/4 |
|
|
Interstitial fluid is what fraction of extracellular fluid? |
3/4 |
|
|
What is a simple question that the Vd is really asking? |
"Where is the drug?" If most is in the plasma, then the Vd is low, if most is outside the plasma then Vd is high. |
|
|
A drug has a volume of distribution that equals 4000 L. Where is the drug most likely in the body? |
Co has to be low so it is outside the plasma being sequestered in the tissue. |
|
|
What units might Vd be presented in? |
L or L/kg |
|
|
What does "Redistribution" mean? |
It means that, in addition to a drug crossing the BBB, lipid-soluble drugs can redistribute into fat tissues prior to elimination. |
|
|
What is thiopental used for? |
Induce anesthesia |
|
|
Thiopental reaches the brain in less than a minute and has a rapid recovery. Why does it have a half-life of nine hours? |
Due to redistribution |
|
|
What does biotransformation mean? |
Drug metabolism |
|
|
True or false: The role of biotransformation is to always inactivate drugs by making them water-soluble and more readily excreted. |
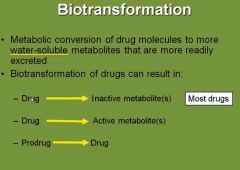
False. |
|
|
Give an example where a drug undergoes biotransformation in the liver and produces active metabolites. |
Benzodiazepines. |
|
|
What does phase I of biotransformation include? |
Modification of a drug molecule via oxidation, reduction or hydrolysis (nonmicrosomal metabolism) and CYT P450 (microsomal metabolism). |
|
|
Cytochrome P450 isozymes contain ____ families and about ____ different enzymes. |
13; 100 |
|
|
P450 have an absolute requirement for what? |
Oxygen and NADPH |
|
|
What family of CYP450 enzymes are considered the most important family and metabolizes about 60% of all known drugs today? |
CYP450 3A4 family |
|
|
What does it mean that a drug or molecule can induce P450 enzymes? Use an example. |
Aromatic hydrocarbons in smoke can induce (increase production) the family of enzymes called 1A2. This means that drugs that share this enzyme pathway of biotransformation are going to be metabolized more rapidly if you are concurrently smoking. |
|
|
A long term smoker has a higher than normal 1A2 family of CYP450 enzymes in his liver. What other drugs would be metabolized more quickly due to his smoking habit? |
(1) Theophylline |
|
|
A smoker has been on dosage-adjusted theophylline for some time. She is convinced to stop smoking. What dosage adjustment is required to be made now? |
Lower theophylline dosage |
|
|
What does it mean that a drug or molecule can inhibit P450 enzymes? Use an example. |
Quinolones or macrolides can inhibit 1A2 family of CYP450 enzymes. This means that drugs that share this biotransformative pathway can possibly reach toxic levels in an individual. |
|
|
What family of CYP450 enzymes would be induced in a patient with chronic bronchitis? |
CYP450 1A2 |
|
|
What are the classes of useful antimicrobial drugs? |
(1) Penicillins |
|
|
Mechanism of action of macrolides? |
They bind irrversibly to a site on the 50S subunit of the bacterial ribosome, thus inhibiting the translocation step of protein synthesis. |
|
|
Macrolides are considered to be ___________ (bacteriocidal/bacteriostatic). |
bacteriostatic, however at higher doses are considered bacteriocidal. |
|
|
This drug is eff ective against many of the same |
Erythromycin (macrolide). Can be used in patients who are allergic to penicillins. |
|
|
List some macrolides. |
(1) Erythromycin |
|
|
A woman with chronic bronchitis is taking theophylline. You prescribe her macrolide antibiotics for her pneumonia. What should you be thinking about? |
Macrolides are inhibitors of 1A2 which theophylline is metabolized by. They may increase theophylline plasma levels. |
|
|
What macrolide should you have in mind as an inhibitor of P450 isozymes (1) and what has almost no P450 inhibitory effect (2)? |
(1) Erythromycin |
|
|
Give an example of a drug that is inhibited in its metabolism by grapefruit juice. |
Statin drugs. |
|
|
A man experiences muscle pain and a change in the color of his urine after having started statin drugs and a new healthy diet. What might be going on? |
Grapefruit juice --> Inhibits statin metabolism --> Toxic levels of statin drugs --> Rhabdomyolysis |
|
|
Hydrolysis involves what enzymes? |
Esterases and amidases (addition of H2O to break bonds) |
|
|
Genetic polymorphisms exist with these hydrolases. What significance does this have? |
Pseudocholinesterases. |
|
|
What endogenous molecules are metabolized by monoamine oxidases? |
(1) Dopamine |
|
|
What exogenous molecule(s) are metabolized by monoamine oxidases? |
(1) Tyramine |
|
|
Tyramine is found in what foods? |
Beer, red wine, cheese, fish |
|
|
What category of enzyme metabolizes aldehydes to acids in ethanol metabolism? |
Dehydrogenases |
|
|
Active components in grapefruit juice include furanocoumarins capable of inhibiting the metabolism of many drugs. Mention 4. |
(1) Alprazolam |
|
|
Equation for maintenance dose? |
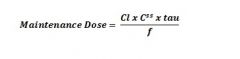
|
|
|
Equation for infusion rate? |
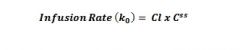
|

