![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
--- Simple Uncatalyzed Reaction ---
From the law of *** *** the reaction velocity is given by: v = k[S]. |
mass action
|
|
|
--- Enzyme Kinetic Study ---
1) *** concentration is varied. 2) *** concentration is constant. 3) The effect on reaction *** is studied. |
1) Substrate
2) Enzyme 3) velocity |
|
|
1) [E0] (free enzyme at *** ***) which is equal to [ET].
2) [ET] = [E]+[ES] --- is called the *** equation. 3) The *** assumption [ES] at any given time will be... k1[E][S] = k-1[ES] |
1) zero time
2) conservation 3) equilibrium 4) |
|
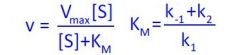
What equation is this?
|
Michaelis-Menten equation
|
|
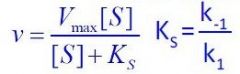
What equation is this?
|
Steady-State equation
|
|
|
1) An alternative to the Michaelis-Menten
model is the.... 2) This model assumes that... |
1) steady-state assumption
2) over time ES levels reach a steady state |
|
|
A comparison of *** values may provide information about the amount of enzyme in different tissues.
|
Vmax
|
|
|
Vmax depends on the amount of *** used whereas KM does not.
|
enzyme
|
|
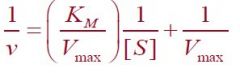
What equation is this?
|
Lineweaver-Burk Equation
|
|
|
Briggs and Haldane came up with what?
|
Steady-State Assumption
|

