![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
60 Cards in this Set
- Front
- Back
|
When describing any wound make sure include
|
AREA SPECIFIC information
- 'special areas' e.g. burns - if increased likelihood of infection - hand, foot, punch bite mechanism - ALL local structures - cosmetic sensitivities |
|
|
Microbiology of animal bites (cats/dogs)
|
Pasteurella
Metronidazole Staph Strep |
|
|
Rabies - how is a suspicious bite treated
|
IMMUNOGLOBULIN
Per kilo dosing Infiltrate wound as much as possible (if extensive, then dilute in N/S to infiltrate all wounds adequately) then into closest limb or gluteal region IM VACCINATION Given as far away as possible from site, 4-5 doses (5 if immunocompromised, or lyssavirus - Australian bat) 0, 3, 7, 14 and 28-30 into deltoid, IM |
|
|
What are the four features of mass effect on CT?
|
Midline shift
Sulcal effacement Ventricular effacement Loss quadrigeminal plate |
|
|
Vasogenic Cf. cytotoxic oedema??
|
x
|
|
|
Ring enhancing lesion mneumonic
|
•D - demyelinating disease
•R radiation necrosis or resolving haematoma •M - metastasis •A - abscess •G - glioblastoma multiforme •I - infarct (subacute phase) •C - contusion •L - lymphoma |
|
|
SIRS, sepsis, severe sepsis, septic shock definitions
|
SIRS - 2 or more of
HR >90, T >38, <36, RR >20 pCO2 <32, WCC >12, <4 Sepsis - above plus infection Severe - above plus organ dysfunction Shock - above plus unresponsive BP to 500mL NS |
|
|
ARISE study
|
Australian RCT currently underway to validate EGDT in our population. Observational study already completed.
|
|
|
When reviewing CXR must always check...
|
IF BREAST SHADOWS ARE PRESENT!!
|
|
|
In returned travellers
1. threshold for admission/observation should be... 2. 3 key diseases to consider 3. 4 main presentations |
1 low
2 malaria, typhoid, dengue 3 diarrhoea, respiratory, neurological, hepatitic |
|
|
5 key features on history
|
itinerary
exposures incubation period prophylaxis taken (including immunisations) and any rx (e.g. antibiotics) features of serious sepsis |
|
|
A purpuric rash in a returned traveller - consider
Jaundice consider... |
meningococcal sepsis, DHF, rickettsia, typhoid, haemorrhagic fevers
hepatitis e.g. viral; also cx of severe infections e.g. malaria |
|
|
Significance of eosinophilia
|
The higher the grade of eosinophilia, the more likely a travel-related diagnosis, particularly helminth infestation (e.g. strongyloidiasis, filariasis, hookworm, schistosomiasis, cutaneous larva migrans and ascariasis).
|
|
|
Blood cultures are most useful for
|
typhoid
|
|
|
Serology may be useful for
|
CMV, EBV, HIV, Hep A, B, C, D, E
Dengue |
|
|
Fever without focus - what are the priorities?
|
Do not miss serious early bacterial infection!
1. identify the seriously ill and treat including empirical therapy 2. identify the 'at risk' patient 3. seek localising signs and symptoms - may not be present for 24-36 hours; can be false localising features e.g. isolated vomiting |
|
|
Urgent and sometimes occult infections...
|
● Bacterial meningitis.
● Meningococcaemia. ● Falciparum malaria ● Febrile neutropenia ● Post splenectomy sepsis. ● Infective endocarditis ● Toxic shock syndromes. ● Necrotizing soft tissue infections. ● Space occupying infections of the head and neck, (such as intracranial abscess and retropharyngeal abscess) |
|
|
At risk patients - detail
1 host characteristics 2 epidemiology 3 non specific features 4 rapidity of evolution |
1. young, elderly (even >50!!), ETOH pt (may be withdrawal), IVDU, DM, febrile neutropenia, splenectomised, immunocompromised
2. epidemiology - overseas visitor/traveller, animal contacts, sick contacts, location spec outbreaks 3. Nonspecific features - myalgia - staph/strep, myositis, nec fas - vomiting - esp with HA or AP, common feature CNS infection/occult sepsis - severe HA - cerebral abscess, bact meningitis, pneumonia - petechiae OR atypical rash - meningococcus until proven otherwise - jaundice - viral hepatitis, liver abscess, malaria, severe bacteraemia - severe sore throat - RPA, epiglottitis - rigors - MAY be viral, more commonly bacterial abscess, bacteraemia, IE, cholangitis, pyelonephritis Evolution - rapid evolution concerning - early presentations (24-48) have less obvious sg/sx |
|
|
Malaria T/F
1 Falciparum has a liver phase 2 Falciparum may present as late as 6/12 especially if on prophylaxis 3 knowlesi is a newly emerging, more benign form of malaria 4 Vivax and oVale have Very persistent hypnozoites and require additional primaquine therapy 5. taking prophylaxis excludes the possibility of malaria 6. cough and diarrhoea may be prominent false localising features |
1 F
2. T 3. F - may be fatal 4. T - malariae, falcip do not 5. F 6. T |
|
|
Severe malaria features...
|
1. Clinical features
● CNS features: Altered consciousness, seizures. ● GIT: Vomiting, jaundice. ● Respiratory distress: An ARDS type picture can occur. ● Renal: Acute renal failure, oliguria. ● CVS: Circulatory collapse ● Hematological: Coagulopathies with spontaneous bleeding. 2. Biochemical features ● Acidosis. ● Severe anemia. ● Hypoglycaemia. 3. Microscopy features ● A parasite count above 100 000/mm3 Or ● >2% of red blood cells parasitised The following groups are also at particular risk of severe malaria: ● Extremes of age, (the very young and the elderly) ● Pregnant women. ● Splenectomized patients. |
|
|
Investigations
T&T - when, how many? ICT - what? |
Q12H, 3 sets
ICT - immunochromatograph test - detects a protein produced by falciparum and also a 'pan malaria protein', v sens and specific PCR to identify species (epidemiological reasons) |
|
|
In suspected malaria, should empirical therapy be given
|
Plasmodium falciparum must be assumed to be the infective agent in the first instance and empirical treatment for this should commence immediately if the diagnosis is confirmed or suspected. Mortality in severe cases will be directly related to delay in the initiation of treatment.
|
|
|
Rabies - closest locations to Australia?
|
Lyssavirus in Royal Botanic gardens!!
Rabies virus in India, Philippines, Thailand, Indonesia (inc Bali) Viruses are closely related members of genus Lyssavirus (RNA rhabdovirus) |
|
|
Regarding Rabies which is incorrect
1. once the virus has entered the CNS, prophylaxis is futile and the disease will be fatal 2. travels at approximately 12-24mm/d along peripheral spinal nerves to enter ganglion 3. from ganglion travels at 200-400mm per day to CNS, and causes encephalitis there, after which it spread outwards via all peripheral nn to all tissues, including salivary glands 4. Australian bat lyssavirus is confined to the fruit bat population 5. in developed countries the hosts are wild animals, undeveloped they are usually domestic 6. incubation period is usually 3-8 weeks, may be 9 days or seven years!! This is modified by site and site of inoculum and host factors |
2. spinal ganglion entry is marked by onset of pain/paraesthesia at site of inoculum
4. NO - all Australian bats may have the potential to carry and transmit the virus |
|
|
Regarding rabies which is correct
1. dogs and cats must be clinically unwell to be infectious 2. onset of symptoms to death is usually months 3. pain or parasthesias at the bite site during the prodromal period may be the only specific symptoms of rabies 4 encephalitis, brainstem dyfunction (spasms, autonomic dysfunction, cranial nerve dysfunction) and multi organ failure are an indication for immunisation 5 viral antigen detection, negri bodies seen on staining, cell culture or serology should be identified prior to treatment |
1 infectious up to a week prior to signs
2 mean of 16 days 3 CORRECT 4 death virtually assured, treatment should have occurred well before this 5 treatment commences prior to microbiological diagnosis Once the disease occurs there is no universally successful treatment for rabies and care of established infection is entirely supportive. There are some case reports of survival with some protocols but these have also had failures. Prognosis remains very poor. Prognosis is good however when immuno-therapy is commenced prior to the onset of CNS symptoms. |
|
|
What is the role of exposure prophylaxis for rabies?
|
The decision to offer post-exposure prophylaxis to a potentially exposed person can be complex and should be made in consultation with the Health Department. If post-exposure prophylaxis is indicated, the Department of Human Services will arrange for rapid delivery of vaccine and immunoglobulin as required.
Particular concern to Australians includes: ● Returned travellers form endemic areas including Bali, who have sustained a bite from any animal. ● Within Australia, bites sustained from bats. Within Australia pre-exposure vaccination should be recommended to those people whose occupation or recreational activities place them at increased risk of being bitten or scratched by a bat. Pre-exposure vaccination should also be recommended for travelers (in particular children who may not reliably report animal bites), who will be spending prolonged periods (i.e. more than one month) in rural parts of rabies endemic areas. |
|
|
What is post exposure treatment for those bitten or scratched when not previously vaccinated?
|
Post-exposure treatment for persons bitten or scratched when not previously vaccinated against rabies:
Effective post-exposure prophylaxis depends on the timely and thorough application of 3 measures. These measures are indicated irrespective of the time which has elapsed since the bite, even if it is weeks or months. The 3 measures are: ● Wound care ● Rabies Immunoglobulin - not necessarily if just a superficial scratch ● Rabies Vaccine First Aid: ● Proper cleansing of the wound is the single most effective measure for reducing the transmission of classic rabies virus. ● Where a person has been injured by a potentially infected animal, the wound should be washed thoroughly for approximately five minutes as soon as possible with soap and water. ● If available, a virucidal antiseptic such as povidone-iodine, iodine tincture, aqueous iodine solution or alcohol (ethanol) should be applied after washing. ● Exposed mucous membranes such as eyes, nose or mouth should be flushed well with water. |
|
|
How is IG and rabies vaccine given?
|
Rabies immunoglobulin:
Human rabies immunoglobulin (HRIG) should be given as a single dose at the same time as the first dose of the post-exposure vaccination course. Vaccine should be commenced at the same time as RIG is given. It should be given into the deltoid muscle at a site distant from the immunoglobulin. Post-exposure prophylaxis for persons not previously immunized against rabies consists of four - five doses of 1.0 mL of rabies vaccine given as deep subcutaneous or intramuscular injection, on days 0, 3, 7, 14 and 28-30. |
|
|
Other concerns with rabies?
|
Tetanus
Antibiotics if secondary infection Quarantine Notification |
|
|
Why are typhoid and paratyphoid labelled 'enteric fevers'?
What is their most important mode of pathology? Most prominent symptomatology? Organisms responsible? |
Enteric portal of entry
Systemic septicaemic disease GIT symptoms Salmonella typhi and salmonella paratyphi |
|
|
What are the complications of typhoid infection?
Which are vaccine preventable? |
1. Death may occur from:
● GIT fluid loss. ● Septic shock. ● GIT perforation. ● GIT hemorrhage. 2. Relapse: ● Relapse of typhoid fever after clinical cure is not uncommon even in immunologically normal individuals. 3. Chronic carriage: ● Chronic carriage of salmonellae is defined as excretion of the organism in the stool for more than 12 months after the acute infection. ● Rates of chronic carriage after S. typhi infection range from 1 to 6 percent, and are higher in patients with cholelithiasis or other biliary tract disease. ● Chronic carriers do not develop recurrent symptomatic disease. They are chronically colonized, and may excrete large numbers of organisms, however have high levels of systemic immunity and do not develop clinical disease. ● Chronic carriers represent infectious risk to others, particularly if involved in food preparation. For this reason, eradication of carriage is usually attempted once such individuals are identified. None - no vaccine 100% |
|
|
Regarding typhoid fever
1 how is it transmitted (risk factors) 2 incubation period 3 reservoir/hosts |
1 ingestions contaminated water/food, including ice, raw vegies, salad, uncooked shellfish
2 8-14 d (a 'week 2' infectious disease) - paratyphoid week1 3 humans |
|
|
Regarding typhoid T/F
1. fever and relative bradycardia are classic, tachycardia may also occur 2. flu like constitutional symptoms are common early 3. GIT syndrome presents with constipation more commonly than N, V and D 4. rose spots are probably embolic, nondiagnostic, and absence does not exclude typhoid 5. A constitutional phase, then transient GI syndrome, is usually in 2nd and 3rd weeks by systemic illness 4. rose spots occur diffusely across the trunk and limbs as a nonblanching rash 5. |
3. NO other way around
5. Yes - delirium, abdo distension, splenomegaly, cardiac symptoms, meningitis, pancreatitis, resp sx and osteomyelitis may occur The classically described time course of the illness is divided into 3 phases, the first week being characterized by fevers and constitutional symptoms, the second by abdominal symptoms and the third week by lethal abdominal complications. It should be noted however that in practice this can be extremely variable. In the absence of acute complications, or death from overwhelming sepsis, resolution is protracted occurring over a period of weeks to months. |
|
|
Typhoid fever
1. how is dx made 2. AB's of choice? 3. Other than resuscitation, AB's, and treatment of any complications - other mx to consider? |
Diagnosis is made by culture of typhoid or paratyphoid bacilli from the blood, urine or faeces.
Serology in the form of the older “Widal” test is no longer routinely used. It measured agglutinating antibodies against H and O antigens of S typhi. High false positives and negatives Reduced susceptibility to fluoroquinolones is common in infections acquired in the Indian subcontinent and Vietnam. Initial empiric choices include: ● Ciprofloxacin, orally or IV if unwell. ● IV ceftriaxone ● IV azithromycin. Follow up to ensure no LT carriage Notification Work exclusion to food handlers/HCW, school children |
|
|
Influenza
1. Types 2. Define shift and drift |
Subdivided by core antigen
A - further divided by surface glycoproteins, haemagglutinin (H), neuraminidase (N). Displays shift and drift - below. humans, birds, pigs and horses. B - only humans C - mild illness in humans Shift - abrupt major change in influenza A virus, may result in pandemic. Occasional, unpredictable Drift - small changes continually over time, A and B Shift Drift |
|
|
Influenza T/F
1. diagnosis is made only after PCR 2. precise dx is not important 3. viral pneumonia should be assumed to be influenza initially 4. almost exclusively benign |
1. The diagnosis is often made “presumptively” in the first instance. This may be possible when there are clear epidemiological indicators, however the diagnosis cannot definitively be made in this manner and the regular occurrence of “sporadic” cases makes diagnosis even more problematic.
The vital consideration as in any PUO, is to consider the following factors carefully, (see also PUO in Adults guidelines and Fever of unknown origin in children guidelines) ● Are there any indicators of possible serious underlying bacterial infection? ● What risk factors does the patient have? ● Is the patient a “returned traveller?” 2. With the periodic emergence of more virulent strains such as Avian Influenza more vigilance is required in the precise diagnosis of “the flu”. 3. If the clinical picture is of a viral pneumonia, could the cause be something other than influenza, such as SARS or varicella-zoster? 4. Although usually thought of as a “benign” illness, it can be fatal in the elderly or immunocompromised or with the emergence of a new severely pathogenic strain. |
|
|
Complications influenza
|
1. Primary viral pneumonia.
2. Secondary bacterial pneumonia: ● Most commonly, Staphylococcus aureus, Streptococcus pneumoniae and Haemophilus influenzae. 3. Exacerbation of asthma, in asthmatics. Very rarely: 4. An acute encephalopathy. 5. Pericarditis / myocarditis |
|
|
Period communicability
|
adults 3-5 from symptoms, children up to 7
|
|
|
Influenza Sx
|
1. Onset of illness is usually very abrupt.
2. Fever is variable, but can be impressively high. 3. Non specific URTI symptoms are common, including: ● Cough ● Sore throat ● Coryza 4. Non specific “constitutional” symptoms include: ● Myalgias, (of the limbs and back in particular) ● Headache ● Nausea and vomiting ● Lethargy and malaise (can be extreme) 5. Lower respiratory symptoms: ● Dyspnea ● Productive cough. ● Pleuritic chest pain. 6. Note that in some cases diarrhea may also be prominent. |
|
|
Investigation for influenza
|
PCR testing allows for the rapid identification of viruses. This test is far more useful than serology testing when rapid identification is required.
Viral PCR studies for respiratory tract infection may be done on nose and throat swabs or Nasopgaryngeal aspirates. Samples should be placed in viral transport medium and sent immediately to pathology. The routine panel of viral testing includes: ● Influenza A & B ● Parainfluenza 1, 2 and 3 ● RSV ● Adenovirus ● Picorna virus. Picornaviruses are divided into two groups, the Enteroviruses (which include Poliovirus, Coxsackievirus and Echovirus) and the Rhinoviruses. If other specific viruses are being sort, or if a severely pathogenic strain is suspected, they should be specified in the request as well as discussed with pathology. The most urgent current examples would include suspected Avian or Swine strains of influenza or the SARS. Results in 24 hours |
|
|
Role of testing
|
Initially to establish that influenza is present in population. A presumptive diagnosis and management of “probable influenza” may then be made providing:
● Respiratory illness and symptoms are mild ● There is no suspicion of possible severe alternative disease. ● Epidemiological grounds have been established. ● The patient is not immunocompromised ● There is no possibility of a severe strain of influenza: ♥ No recent travel to an area known to have such strains ♥ No recent contact with documented cases of severe strains |
|
|
Severe or potentially severe cases - admit if...
|
● Other potentially serious illness, including bacterial disease cannot be excluded especially when the patient appears unwell.
● Recent travel from a region known to be experiencing outbreaks of severely pathogenic strains. In particular where there has been a history of travel in a country currently known to have avian influenza circulating in poultry. ● Contact with a confirmed case of known severely pathogenic strains ● Patients who are immunocompronised. ● The elderly or very young should not be “labeled” influenza on clinical grounds alone. |
|
|
Mx issues to consider
|
1. Supportive
● Oxygenation is critical and ventilatory support may be required. 2. Barrier nursing precautions. A. Isolation nursing, (including specialist isolation “negative pressure” rooms). ● Suspected cases should not be left in waiting rooms. B The patient should be given a “high filtration” facemask. C Staff should wear facemask, gloves, gowns as well as suitable eye protection. 3. Specific antiviral treatment. 4. Notification |
|
|
Specific antivirals
- benefits - who gets them - timing and administration |
● Reduce the duration of symptoms
● Reduce the severity and complications ● Reduce infectivity of infected patients. ● Help prevent infection in uninfected subjects. They should be considered ● During outbreaks within institutions, such as nursing homes or hospitals, or where people are at high risk for complications and are in close contact with each other. ● immunocompromised. ● unwell. ● infected or potentially infected with known severe strains. To be effective therapeutically (as opposed to prophylactically) these antiviral agents need to be given within 48 hours of the onset of symptoms. Oseltamivir 75 mg orally, twice daily for 5 days. |
|
|
Dengue fever
1. mosquito 2. DHF - when does it occur? 3. incubation period 4. areas found |
1 Aedes egypti and albopictus
2. re-exposure to different serotype e.g. immune enhancement (4 exist) 3. 3-14 d 4. North QLD, tropical regions of world |
|
|
Dengue t/f
1. person to person transmission? 2 infection with one serotype results in LT immunity to that serotype 3. classic dengue has a higher rate of mild subclinical disease in children cf adults 4. DHF mainly children |
1 does not occur
2 t 3 t 4 t |
|
|
Classic dengue clinical features
|
1. Dengue fever classically presents as an acute febrile illness of sudden onset.
2. Fever lasting three to five days 3. Non-specific constitutional symptoms: These can be extremely debilitating and are usually described as “flu-like” ● Myalgias (particularly backache). ● Arthralgias ● Headache, frontal and retro-orbital pain is common. ● Lethargy/ malaise. 4. GIT upset ● Anorexia, nausea and vomiting. 5. Rash ● A non-specific viral rash may be seen in about 50% of cases. 6. Convalescence may be prolonged. |
|
|
DHF which is incorrect
1. may result in dengue shock syndrome, high fatality rate 2. mortality up to 5% even without DSS 3. haemorrhagic phenomena differentiate from classic syndrome 4. haemorrhagic complications are more common than DSS 5. key Ix findings are thrombocytopenia and elevated haematocrit; IgM confirms dx but may take 5 days to rise, PCR is available for blood/CSF |
Haemorrhagic phenomena
● Haemorrhagic skin lesions, petechiae, purpura. ● Mucosal bleeding, gums, epistaxis or GIT ● Thrombocytopenia. Check for a positive tourniquet test. The tourniquet test is performed by inflating a blood pressure cuff on the upper arm to a point midway between systolic and diastolic blood pressures for five minutes. A test is considered positive when there are 20 or more petechiae per square inch (6.25 cm2) on the forearm. 4 INCORRECT - DSS is more common (in particular hypotension; endpoints are circulatory collapse and death) |
|
|
Management
|
1. Supportive treatment:
There is no specific treatment for dengue fever. ● Fluid resuscitation is important, especially in cases of dengue hemorrhagic fever. ● Inotropes may be required for cardiogenic shock ● Other supportive measures as clinically indicated. 2. Anti-pyretics: ● Paracetomol may be given. Aspirin and NSAIDs are best avoided due to the potential to aggravate bleeding complications. 3. Monitoring: ● Platelet counts and hematocrit determinations should be carefully monitored to allow prompt recognition of the development of DHF and institution of fluid therapy. 4. FFP and platelets may be required in cases of DIC. 5. Notification |
|
|
The asplenic and hyposplenic patient which is incorrect
1. is at risk of serious fulminant sepsis (OPSI - overwhelming post splenectomy infection) 2. lifelong risk, highest in children and immunocompromised 3. compliance with prophylaxis is poor 4. the Victorian spleen registry VSR exist to educate and reduce risk post splenectomy 5. OPSI has mortality up to 10% |
5. INCORRECT - up to 50%
The threshold to investigate, initiate empirical treatment to cover serious bacterial infection and to admit must be low in these patients. |
|
|
Which organisms are possible?
|
1. Pathogenic encapsulated organisms:
● Streptococcus pneumoniae: ♥ This is the commonest cause, (responsible for > 50 % of cases of overwhelming post splenectomy sepsis). ● Neisseria meningitidis (the meningococcus) ● Haemophilus influenzae type b (Hib) 2. Plasmodium protozoa, (i.e. malaria): ● There is an increased susceptivity to intraerythrocytic parasites in general. 3. Capnocytophaga canimorsus: ● There is an increased risk of severe sepsis due to this agent in patients with asplenia or hyposplenia who are bitten or even just scratched by dogs or cats. Less commonly: 3. Gram negative bacilli 4. Streptococcus suis 5. Bordetella holmesii 6. Babesiosis: ● This is an unusual infection transmitted by ticks. It can be fatal in splenectomised patients. It has not to date been described in Australia or New Zealand. |
|
|
Splenectomised patients which is incorrect
1. OPSI may present with mild nonspecific, nonlocalising symptoms 2. a laparotomy scar or evidence of haematological disease (hepatomegaly, lymphadenopathy) may be the only evidence of splenectomy/functional asplenia 3. Howell-Jolly bodies (normally removed by spleen) on blood film and reduced levels of IgM memory B cells (predicts impaired immune response) act as indicators of asplenia or hyposplenia 4. patients should have a standby supply of antibiotics with instructions on when to commence 5. advice includes the traveller's risk of severe malaria, and early and optimal rx of animal wounds 6. daily prophylaxis is taken lifelong post splenectomy 7. annual vaccinations for pneumococcal, meningococcal, HIB, influenza are required |
An under functioning spleen may be due to:
● Subtotal splenectomy, (rarely) ● Some autoimmune diseases; Coeliac disease in particular. ● Following bone marrow transplantation. ● Haemoglobinopathies, including: ♥ Sickle cell anaemia ♥ Thalassaemia major 6. INCORRECT - minimum 2 years post splenectomy, usually augmentin or phenoxymethylpenicillin |
|
|
Septic shock - what does the history entail?
|
1. A thorough history is often the most useful guide to the likely source of sepsis.
2. It is important to establish if there are any risk factors for depressed immunity, including: ● Does the patient have HIV? ● Is the patient an oncology patient, in particular are they receiving radiotherapy or chemotherapy, (suspect febrile neutropenia) or on any immuno-depressant drugs. ● Does the patient have Addison’s disease? ● Other significant co-morbidity, diabetics, chronic renal failure, chronic liver failure. 3, Source ● Overseas travel: falciparum, malaria needs to be considered ● IV access ports or surgical devices, ● structural cardiac disease ● important “epidemiological” factors, eg, recent contact with infectious disease, such as meningococcus or recent legionella outbreaks? ● recent surgery? |
|
|
Define SIRS
|
2 or more of the following:
● Tachypnoea, (RR > 20 / min), or a PaCO2 < 32 mmHg, or a minute ventilation value of > 10 L / min, where the patient is intubated and spontaneously breathing. ● Tachycardia, (> 90 beats per minute) ● A core body temperature of > 38 C or < 36 C. ● A WCC of > 12,000 cells / micro L or < 4000 cells / micro L |
|
|
EGDT
1. fluid resuscitation - type, amount, endpoints |
Crystalloids are given, usually normal saline
● In adults, generally 2-3 liters can be given. ● If there is insufficient response from this initial fluid resuscitation, then the commencement inotropic support needs to be considered, N or A no outcome difference yet shown UO > 0.5 mls / kg / hr Central venous pressure monitoring: Aim for a minimum CVP of 8 mmHg, (>12 mmHg for ventilated patients) Arterial line: Aim for a mean arterial pressure of at least 65 mmHg. |
|
|
Compare septic, cardiogenic and hypovolaemic shock by cardiac index, CVP, systemic vascular resistance and O2 delivery
|
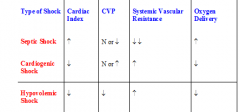
|
|
|
Choice of antibiotics for
- severe pneumonia - febrile neutropenia - severe sepsis without source |
Severe pneumonia:
1 ceftriaxone 1 g IV, daily OR 2 benzylpenicillin 1.2 g IV, 4-hourly PLUS gentamicin 4 to 6 mg/kg (severe sepsis: 7 mg/kg) IV OR 2 cefotaxime 1 g IV, 8-hourly 3 if immed pen hypersens moxifloxacin PLUS WITH EACH OF THE ABOVE REGIMES azithromycin 500 mg IV, daily Febrile neutropenia: 1 cefepime 2 g (child: 50 mg/kg up to 2 g) IV, 8-hourly OR 1ceftazidime 2 g (child: 50 mg/kg up to 2 g) IV, 8-hourly OR 1 piperacillin+tazobactam 4+0.5 g (child: 100+12.5 mg/kg up to 4+0.5 g) IV, 8-hourly. Severe sepsis without source: di/flucloxacillin 2 g IV, 6-hourly PLUS gentamicin 7 mg/kg IV Cephazolin if pen sensitivity, or vanc if immediate pen sens. Add benpen if meningococcal suspected |
|
|
Other adjuncts to septic shock treatment
|
TREAT SOURCE - surgery
Maintain oxygenation. ARDS type ventilation if ETT required Normoglycaemia Early referral for dialysis if ARF HB 70 -90 Persistent hypotension -> Vasopressin may be considered as an adjunct to noradrenaline or adrenaline. The dose is 0.01 - 0.04 units / minute. If vasopressin is to be used, then there is some evidence that steroids should also be given before vasopressin is commenced. Nutritional support within 48 hours, enteral route best |
|
|
What is the indication for steroids in septic shock?
|
Stress dose IV hydrocortisone (100mg bd) should be given if the patient is already on or has recently been on steroids or if the patient is known to have Addison's disease or has bacterial meningitis.
no proven benefit for the routine use of steroids in septic shock. |
|
|
No benefit in septic shock for...
|
Activated protein C
Albumin infusion Bicarbonate High dose steroids (unless cerebral abscess) Renal dose dopamine |

