![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
85 Cards in this Set
- Front
- Back
|
Describe the mechanisms of bacterial resistance to Beta-lactam antibiotics.
|
* Destruction of the antibiotic by Beta-lactamases (most important and most common)
* Decreased affinity of antibiotic for PBPs (major mechanism of MRSA resistance) * Decreased penetration of the antibiotic to the site of action * Increased efflux |
|
|
Describe the mechanisms of bacterial resistance to Aminoglycosides.
|
* Aminoglycoside-modifying enzymes (major mechanism)
* Altered drug uptake * Altered ribosomal binding site (rare) |
|
|
Describe the mechanisms of bacterial resistance to Fluoroquinolones.
|
* Chromosomal mutations in DNA gyrase and topoisomerase IV (most clinically important)
* Expression of efflux proteins |
|
|
Describe the mechanisms of bacterial resistance to Macrolides.
|
* Alterations of ribosomal binding site
- single point mutation of the 50s ribosomal subunit - Methylation of the 50S ribosomal subunit causes high-level resistance (most important mechanism) * Efflux across the cell membrane (most common) * Decreased membrane permiablility among gram - bacilli * Enzymatic inactivation of erythromycin among gram - bacilli |
|
|
Describe the mechanisms of bacterial resistance to Vancomycin.
|
* Alterations in sunthesis of cell wall precursors:
D-alanyl-D-alanine --> D-alanyl - D-lactate (Most important and most common mechanism) |
|
|
Describe mechanisms of resistance employed by Staphylococcus aureus.
|
* Beta-lactamase production = penicillinases
* Alterations in PBPs (most important - eliminates the beta-lactams for treatment) * Topoisomerase mutations (fluoroquinolone resistance) * Efflux across cell membrane (minor - affects multiple classes) * Resistance genes often carried in groups or "cassettes" affecting multiple drug classes |
|
|
Describe mechanisms of resistance employed by Streptococcus pneumoniae.
|
* Alterations in PBPs
- may result in low or high level resistance - Most clinically important * Efflux proteins - macrolides and clindamycin * Ribosomal methylation - macrolides and clindamycin * Topoisomerase IV mutations - fluoroquinolones * Beta-lactamase production (rare) * Resistance to multiple antibiotics common - resistance genes carried in cassettes |
|
|
Describe mechanisms of resistance employed by Enterococci
|
Intrinsic resistance due to:
- PBPs with decreased affinity - Decreased permiability of the cell membrane Acquired resistance - Further alterations in PBPs - Aminoglycoside-modifying enzymes - Alterations in cell wall precursors (VRE) |
|
|
Describe mechanisms of resistance employed by Gram negative bacilli
|
* Beta-lactamase production
- Most common mechanism of resistance, found in most important pathogens - Most important mechanism of resistance - ESBLs are very problematic in certain areas * Decreased permeability of the outer membrane - Especially important in combo wiuth beta-lactamse production * Aminoglycoside-inactivating enzymes * DNA gyrase mutations (gyr A and gyr B) * Efflux pump expression |
|
|
Describe mechanisms of resistance employed by Anaerobes
|
* Beta-lactamase production most common mechanism
- Penicillinases and cephalosporinases - Enzymes usually inhibited by beta-lactamse inhibitors - "Metallo beta-lactamses" confer resistance to carbapenems. Producrd by some strains but still relatively uncommon * Multiple other mechanisms may be present |
|
|
Risk Factors for the Developement of Resistance
|
* overuse of specific antibiotics
* Overuse of antibiotics in general * Use of broad-spectrum antibiotics * Inappropriate drug dosing |
|
|
Structure of Sulfonamides
|
Basic structure includes:
- Amine - Benzene ring - SO2 group * Substitutions at various position have different effects on activity - SO2 group substtitutions affect effectiveness of competitive PABA inhibition - Substitutions on the Amine group decrease oral absorption - The free amine at carbon 4 confers a high degree of antibacterial activity |
|
|
MOA of Sulfonamides
|
* Stuctural analogues of PAPA and Compete with PABA in the Folic Acid Synthesis Pathway
- Folic acid is required for purine synthesis - Humans can use exogenous Folic Acid (supplements) Bacteria cannot so these drugs selectively inhibit bacterial growth * Bind to and Inhibit Dihydropteroate Synthetase (Step 1) * Bacteriostatic |
|
|
Mechanisms of Resistance to Sulfonamises
|
* Intrinsic resistance
- Enterococcus faecalis and lactobacilli are auxotrophic for folic acid * Acquired resistance - Single chromosomal mutation and/or Plasmid mediated resistance - Alteration in dihydropteroate synthetase - Overproduction of PABA - Reduced uptake/efflux pumps |
|
|
PK of Sulfonamides
|
* Good oral absorption 70-90%
* Protein binding is highly variable 35-90% (sulfadiazine ≈ 45%, sulfisoxazole > 90%) * Rapid penetration into all tissues (CSF, Pleural fluid, peritoneal, synovial fluid, transplacental, breast milk) * Elimination: Renal and liver metabolism (acetylation) For most sulfonamides the kidney is responsible for > 50% of unchanged drug removal Crystalluria – occurs with some sulfonamides in acidic urine |
|
|
Metabolism of Sulfonamides
|
* Metabolism occurs primarily in the liver via (N4 Acetylation)
- N4-acetylated sulfa’s do not possess antibacterial activity - N4-acetylated and un-metabolized sulfa’s are excreted and highly concentrated in the urine - N4-acetylated sulfa’s have limited solubility at neutral or acidic pH + Crystalluria + Renal toxicity * Glucuronidation at the N1 sulfonamide nitrogen * Hydroxylation at the N4 position by the CYP 450 2C9 enzyme - Reactive hydroxylamine metabolite is formed through oxidation of the arylamine group - Important in forming the haptenated structure which the body recognizes as foreign |
|
|
Classification of sulfonamides
|
- Short to Medium Acting Sulfonamides:
* highly soluble * rapidly absorbed and eliminated * sulfisoxazole, sulfamethoxazole, sulfamethizole * the half-life of sulfamethoxazole is 11 hours - Long-acting sulfonamides: * few available in the US due to association with Stevens-Johnson syndrome * sulfadoxine has a half-live of 100-200 hours * used in the treatment of P. falciparum - Poorly Absorbed: * little to no absorption from the GI tract * used to “cleanse” bowel prior to surgery * sulfathalidine, sulfaguanidine * Sulfasalazine (Azulfidine®) is used to treat ulcerative colitis. - Prodrug hydrolyzed by bacteria in the gut to yielding 5-aminosalycilate (e.g. mesalamine) and sulfapyridine - Topical: * sulfacetamide is used in ophthalmic ointments and solutions * silver sulfadiazine (Silvadene®) * mafenide (Sulfamylon®) |
|
|
Adverse effects of sulfonamides
|
* Rash
- Mild - Stevens-Johnson Syndrome (SJS) - Toxic epidermal necrolysis (TEN) * Hemolytic Anemia - Glucose-6-Phosphate Dehydrogenase (G6PD deficiency) * Agranulocytosis * Vasculitis * Crystalluria * Fever * N/V * Diarrhea |
|
|
Allergic Reactions
|
* Drug-induced allergic reactions occur in approximately 5% of patients
Allergic process mediators: * IgE mediated – N1 “SO2NH2” group - Maculopapular eruption or urticarial rash - Develops 1-3 days after medication initiation - Anaphylaxis can occur with repeat exposure * Toxic metabolite - N4 Haptenation - Hydroxylamine metabolite (-NHOH) - Nitroso metabolite (-NO) - Primarily responsible for tissue toxicity - Occurs 7-14 days after medication initiation - Fever, rash, erythema multiforme, multi-organ toxicity Highest risk: sulfonylarylamines (Sulfonamide moiety directly connected to a benzene ring with an un-substituted amine (-NH2) moeity at the N4 position) > Non-sulfonylarylamines (Sulfonamide moiety connected to a benzene ring or other cyclic structure without the un-substituted amine moiety at the N4 position) > Group 3 (Sulfonamide moiety not directly connected to a benzene ring) * Rapid/Acute desensitisation is possible in the ICU setting |
|
|
Clinical uses of Sulfonamides
|
* Sulfonamides exhibit in vitro activity against a broad spectrum of gram-positive and gram-negative bacteria, as well as actinomyces, toxoplasma, and Plasmodium spp.
* These agents are bacteriostatic * Unfortunately, resistance to sulfonamides is wide-spread and increasingly common limitting clinical use |
|
|
DHFR Inhibitor to know
|
Trimethoprim
|
|
|
Trimethoprim MOA
|
* Structural analogue of dihydrofolic acid (DHF)
* Binds to the enzyme dihydrofolate reductase (DHFR) and inhibits the transformation of DHF to tetrahydrofolic acid |
|
|
Trimethoprim Mechanisms of Resistance
|
Chromosomal and Plasmid mediated (Tn7 transposon)
* Decreased affinity of DHFR for TMP * Hyperproduction of DHFR * Decreased porin permeability |
|
|
PK of Trimethoprim
|
* Absorption: TMP is absorbed readily and essentially completely following oral administration
* Distribution: TMP is widely distributed in virtually all tissue (CSF levels ~ 50% that observed in serum) * Metabolism: TMP is hydroxylated to inactive metabolites, which are also cleared by the kidney * Elimination: The majority of TMP (60%-80%) is excreted unchanged in the urine - The serum half-life of TMP is 10 to 12 hours (sulfamamoximal) |
|
|
Adverse effects of Trimethoprim
|
* Nausea and Vomiting
* Pancytopenia (decrease in WBC etc) * Renal disorders (Increased BUN and SCr) * Hyperkalemia (Decreased potassium excretion by the renal tubule) |
|
|
Drug-drug Interactions
of concern with Trimethoprim |
* Displacement of bound drug from albumin or decreased metabolism
* Sulfonylurea hypoglycemic agents * Coumadin * Phenytoin * Methotrexate * Azathioprine * Coumadin is the most important one to remember as a the effect on INR is significant |
|
|
Clinical uses of Trimpethoprim
|
* Broad Spectrum (Activity against many aerobic gram positive and gram negative bacteria)
* Resistant organisms limit use (Pseudomonas aeruginosa [always], Atypicals: Legionella, Mycoplasma, Chlamydia, Treponema pallidum (syphilis), Mycobacterium tuberculosis (TB), most anaerobes) * Primarily used as TMP/SMX Combination Therapy - Synergistic effect - Helps overcome reistance * Urinary Tract Infections - Cystitis, Pyelonephritis, Prostatitis * Community acquired MRSA - Cellulitis, etc. * STDs - Gonococcal (high dose required, not 1st line) - Chancroid * Ear, Nose Throat (not first line) * Pneumonia - PCP, Nocardia, stenotrophomonas * Enteric infections - Typhoid fever (salmonella typhi) - Salmonella, shigella, e.coli, vibrio, aeromonas, plesiomonas * Meningitis - L. monocytogenes * Mycoses - Paracoccidioides brasiliensis, PCP * Protozoal Infections - Plasmodium falciparum (malaria) - Cyclospora cayetanensis - Isospora belli |
|
|
What family or class of antibiotics does Rifampin belong to?
|
Rifamycins
|
|
|
What is the basic structure of Rifampin?
|
macrocyclic
|
|
|
Rifampin MOA
|
* Rifampin binds to the β-subunit of bacterial RNA polymerase (DNA-dependent RNA polymerase) and inhibits the initiation of transcription
* Bacteriostatic but bactericidal against M. tuberculosis * Concentration dependent killing – Cmax:MIC ratio or AUC:MIC ratio |
|
|
Mechanisms of resistance to Rifamin
|
* Single mutation in the RNA polymerase beta subunit gene (rpoB)
* Acquired resistance: - Monotherapy leads to rapid selection of resistant mutants * Resistance prevention: - Use multiple antimycobacterial drugs or a second antimicrobial agent - Never use Alone |
|
|
PK of Rifampin
|
* Absorption :
- Good oral bioavailability - Food may delay rate of absorption, but not extent - Rifampin is also available IV * Vd (0.7 L/kg): Distributes into most tissues and fluids - CNS penetration 5-20% * Protein binding 85% * Elimination: - T1/2: 2-4 hours * Autoinduction * De-acetylated and hydroxylated * Parent drug and metabolites are excreted in bile and eliminated in feces * Parent drug undergoes extensive entero-hepatic recirculation * Only 13-24% of rifampin is excreted unchanged in urine * HEPATIC elimination |
|
|
Adverse effects of Rifampin
|
* Hypersensitivity reactions (< 0.5%)
- Thrombocytopenia, ARF, interstitial nephritis, shock, hemolytic anemia * N,V, HA, dizziness * Rash, pruritis, fever * Orange/red discoloration of body fluids: urine, tears, staining of contact lenses (counsel on this) * Hepatotoxic (jaundice) - increased incidence with liver disease, age, malnutrition, acute renal failure |
|
|
Drug Drug interactions of Rifampin
|
* Potent inducer of hepatic cytochrome P450 enzymes
* Most marked effect on CYP450 3A4 and 2C8/9 - Occurs within first days, peaks at 7 days, and persists 7-14 days after dosing stopped - Decreases levels of other drugs Simvastatin Clarithromycin Warfarin Cyclosporine Oral contraceptives Protease Inhibitors |
|
|
Clinical uses of Rifampin
|
* Mycobacterial infections – pulmonary tuberculosis
* Meningitis prophylaxis – meningococcal meningitis, Haemophilus influenzae type B meningitis * Endocarditis – addition to vancomycin and gentamicin for Staphylococcus epidermidis prosthetic valve endocarditis * Osteomyelitis and septic arthritis – may be used in addition to antistaphylococcal drug * Meningitis * Infected cerebrospinal fluid shunts, vascular grafts, and implants |
|
|
Nitrofurantoin Structure
|
* contains a nitro moiety that is responsible for its antimicrobial activity
|
|
|
Nitrofurantoin MOA
|
* Bacterial reductases reduce nitrofurantoin to reactive metabolites
- Metabolites react with nucleophilic sites on bacterial macromolecules and inhibit enzymes of the citric acid cycle as well as DNA, RNA, and protein synthesis * Bacteriostatic at low concentrations, but bactericidal at high concentrations (i.e., concentrated in urine) |
|
|
Nitrofurantoin Mechanisms of Resistance
|
* Reduced nitrofuran reductase activity
(b/c not converted to toxic metabolites) * Changes in cell wall permeability * Resistance not very common 85% of urine pathogens still susceptible * Cross-resistance uncommon |
|
|
Nitrofurantoin PK
|
* Absorption: Bioavailability ~ 90%
- Slower absorption with food or macrocrystalline formulation * Vd: 40 Liters – high concentrations in urine * Protein binding 60% * Elimination - Renal – glomerular filtration + tubular secretion - Rapid renal elimination keep plasma concentrations low - Acidic urine increases reabsorption - Alkalinization decreases reabsorption and decreases effectiveness - Renal excretion is proportional to CrCl, so low concentrations in urine and high plasma concentrations in severe renal impairment * Avoid in patients with CrCl < 60 ml/min |
|
|
Nitrofurantoin Adverse Effects
|
* N/V, HA, dizziness, confusion
* Brown urine * Peripheral neuritis * Pulmonary reactions - Acute pneumonitis - Respiratory infection or pulmonary edema *Allergic reaction - eosinophilia - Interstitial fibrosis * Pancreatitis * Hepatotoxicity - Hepatocellular damage and cholestatic jaundice * Acute hemolysis - G6PD deficiency * Megaloblastic anemia with folic acid deficiency, leukopenia, thrombocytopenia, agranulocytosis, aplastic anemia **Contraindicated in newborns and nursing mothers if G6PD status unknown - May precipitate hemolytic anemia in the newborn |
|
|
Nitrofurantoin Drug drug interactions
|
"Probenicid inhibits tubular secretion lowering urinary concentrations
" "Food may increase absorption " "Concomitant antacids can reduce rate and extent of absorption " |
|
|
Clinical uses of Nitrofurantoin
|
"Uncomplicated urinary tract infections
Prophylactic treatment of recurrent UTIs " "Never used for systemic infections! " "Nitrofurantoin is effective for treating a majority of bacterial urinary pathogens - Utilized for treating uncomplicated UTIs - Avoid use in patients with renal insufficiency " |
|
|
Chloramphenicol MOA
|
"Interferes with microbial protein synthesis by binding to the bacterial 50S ribosomal subunit
" "Bacteriostatic against most organisms " "Bactericidal against : S. pneumoniae, H. influenzae, and N. meningitidis (three most common to cause meningitis " |
|
|
Chloramphenicol Mechanisms of Resistance
|
"Inactivation by acetyltransferase
" "Reduced permeability and uptake Loss of outer membrane proteins " "Efflux pumps " |
|
|
Chloramphenicol PK
|
* Absorption;
- Well absorbed – Cmax 1-2 hours * Distribution: - Good penetration into most tissues and bodily fluids Synovial, pleural, peritoneal, pericardial, aqueous, vitreous - CSF 40-65% of serum - Poor bile penetration * Metabolism: - Hepatic - Conjugated to inactive chloramphenicol glucuronide * Elimination: Renal – 5-10% by glomerular filtration |
|
|
Chloramphenicol Adverse events
|
* Hematologic toxicity
- Inhibits mitochondrial enzymes necessary for heme synthesis - Hemolytic anemia (G6PD) - Myelosuppression - Idiosyncratic aplastic anemia (1 in 25,000-40,000) *Irreversible and fatal *May occur weeks to months after therapy * Gray baby syndrome (used to be used in neonates) - Premature and full term infants – due to decreased hepatic conjugation and excretion - Older children or adults with elevate levels (> 50 mcg/ml) * Neurologic toxicity - Optic neuritis, peripheral neuritis, mental status changes - Decreased visual acuity, loss of vision |
|
|
Chloramphenicol Drug Drug interactions
|
* Inhibits CYP450
2C8/9 and 3A4 * CYP450 inducers may increase chloramphenicol clearance - Rifampin, phenytoin, phenobarbital * CYP450 inhibitors may decrease chloramphenicol clearance - Cimetidine, erythromycin |
|
|
Chloramphenicol Clinical uses
|
* No longer the therapy of choice due to adverse effect – specifically aplastic anemia
* Bacterial meningitis S. pneumoniae, H. influenzae, N. meningitidis * Mult-drug resistant organisms, VRE |
|
|
Chloramphenicol Monitoring
|
* Lab Monitoring:
- Baseline: CBC, SCr, LFTs - Twice per week: CBC with differential - Weekly: Kidney and liver function tests * Chloramphenicol serum concentrations - Patients at high-risk of toxicity - Monitor peak concentrations * 1 hour post IV administration – Peak [] is important * Goal = 10-25 mcg/ml * Concentrations > 25 are associated with bone marrow suppression * Concentrations > 40 are associated with gray baby syndrome |
|
|
Four major mechanisms of antibiotic resistance
|
* Target site modification
* Enzymatic inactivation of drugs * Decreased penetration of cell wall or membrane * Drug efflux |
|
|
Level of antibiotic resistance confered by beta-lactamse production is mediated by numerous factors including...
|
- Type of β-lactamase enzyme
* many β-lactamase enzymes are specific to one or more antibiotics eg. penicillinase or cephalosporinase - Affinity of the antibiotic for the β-lactamase * Some antibiotics with low affinity for the β-lactamase are less effected - β-lactamase stability of the antibiotic * Increased stability means the β-lactamase has less effect - Concentration of antibiotic * drug concentration vs. enzyme concentration, enough drug can overwhelm the β-lactamase - Rate of antibiotic diffusion into periplasmic space (Gram-negatives) * This is "running the guantlet, the quicker the drug moves through the less chance of inactivation - Susceptibility of target PBP to antibiotic |
|
|
S. pneumoniae ______ produces β-lactamases
|
rarely
|
|
|
β-Lactamase Mediated Resistance is particularly important among _________ --> ________ production in > ___% of strains
|
Staphylococcus aureus
penicillinase 90% |
|
|
Resistance genes carried on multi-drug resistance plasmids
called_________ |
Cassettes
|
|
|
Are Enterococci β-Lactamase producers and how important is this mechanism of resistance?
|
Enterococci also occasionally produce β-lactamase, but not a clinically important mechanism of resistance.
|
|
|
Discuss β-Lactamase Mediated Resistance Among Gram-Negative Bacteria
|
* Very complex mechanism of resistance; virtually all gram-negative bacteria produce β-lactamases to some degree
* Over 500 different types of β-lactamases have been described * Enzymes secreted into periplasmic space * May be either: - Chromosomal or plasmid-mediated - Constitutive or inducible * Examples of Gram-Negative β-Lactamase Producers - Plasmid-mediated, constitutive: + Haemophilus influenzae + Moraxella catarrhalis + E. coli + Klebsiella pneumoniae + Proteus mirabilis - e.g. TEM, SHV, ESBLs - Chromosomally-mediated, inducible: + Serratia spp. + Pseudomonas aeruginosa + Acinetobacter spp. + Citrobacter spp. + Enterobacter spp. (SPACE organisms) - e.g. ampC enzymes |
|
|
Constitutive vs. Inducible production of β-Lactamases
|
* Constitutive = constant level of production (either high or low)
* Inducible = expressed after exposure to antibiotic - Among -lactams, level of expression varies with the induction potential of the specific agent - Imipenem = strong inducer - Piperacillin = weak inducer |
|
|
Stable Derepression
|
* Permanent production of large amounts of beta-lactamase independent of antibiotic exposure (“hyperproduction”)
* Caused by mutation in repressor genes which would normally regulate & decrease expression of the enzymes |
|
|
TEM-1, TEM-2, SHV-1 β-Lactamases
|
* Most common plasmid-mediated β-lactamases in gram-negative bacteria
* Often constitutive * Extended-spectrum cephalosporins (2nd, 3rd, & 4th-generations) resist hydrolysis by these beta-lactamases * β-lactamase inhibitors (e.g., clavulanate, tazobactam) protect parent β-lactam compounds and provide improved activity |
|
|
Extended-Spectrum β-Lactamases (ESBLs)
|
* Mutants of classic enzymes associated with minor amino acid substitutions
* Hydrolyze extended-spectrum cephalosporins (including 3rd- and 4th-gen. cephs) and aztreonam * Carbapenems and cephamycins are spared * Usually inhibited by β-lactamase inhibitors (e.g. clavulanate, tazobactam) * ESBLs most commonly produced by Klebsiella and E. coli (Also reported in Enterobacter, Proteus, many others) * Plasmid-mediated resistance facilitates spread - Within and between species - Outbreaks caused by ESBL-producers common in hospitals and larger geographical areas * Significant laboratory detection issues * Therapeutic implications - Associated with multidrug resistance (β-lactams, aminoglycosides, fluoroquinolones) - Carbapenems considered drugs of choice in management of infections caused by ESBL-producers - Piperacillin/tazobactam may be effective but clinical data lacking * Formulary implications : To prevent or address outbreaks |
|
|
β-Lactamase Mediated Resistance Among Anaerobic Bacteria
|
* Very common mechanism of resistance among anaerobes
* May produce penicillinases and/or cephalosporinases * Enzymes usually inhibited by β-lactamase inhibitors (i.e. clavulanate, sulbactam, tazobactam) |
|
|
Resistance to β-Lactams Through Decreased Membrane Permeability
|
* Mechanism not commonly found among gram-positive bacteria
* Important mechanism among gram-negatives, particularly in combination with β-lactamases * Decreased permeability often mediated through changes in porins: - Decreased porin production - Porin mutation - Expression of new porins with altered selectivity |
|
|
Resistance to β-Lactams Through Alterations in PBPs
|
* Particularly common and important mechanism of resistance among gram-positive bacteria
- Staphylococci, Streptococcus pneumoniae, enterococci * PBP alterations may produce either low-level or high-level resistance - Depends on many factors, including actual change in antibiotic binding affinity caused by mutation - e.g. enterococci |
|
|
Resistance to β-Lactams Through Expression of Efflux Systems
|
* Most studied among Gram-positives, especially S. aureus
* Increasingly recognized as important mechanism of β-lactam resistance among Gram-negative bacilli - e.g. Pseudomonas aeruginosa * Often produces relatively low level of resistance when expressed alone - Penetration of antibiotics into cell may overwhelm efflux capacity * Particularly important in combination with β-lactamases and/or porin alterations |
|
|
Heteroresistant VISA (hVISA)
|
When different colonies are picked out and cultured separately, then kill-curve experiments processed one resistant strain may be found hiding amongst all the susceptible strains
|
|
|
Prefered agents for use against Methicillin-Susceptible Staphylococcus aureus
|
Nafcillin, oxacillin
Amoxicillin/clavulanate Ampicillin/sulbactam Ticarcillin/clavulanate Piperacillin/tazobactam Cefazolin (1st gen. cephs) Cefuroxime (2nd gen. cephs) Cefepime Carbapenems Erythromycin Clarithromycin Azithromycin Telithromycin Levofloxacin Moxifloxacin Gemifloxacin Clindamycin Doxycycline Tigecycline TMP/SMX Active but not preferred: Vancomycin Quinupristin/dalfopristin Linezolid Daptomycin Telavancin |
|
|
Prefered agents for use against Methicillin-Resistant Staphylococcus aureus
|
Vancomycin
Linezolid Quinupristin/dalfopristin Daptomycin Telavancin Tigecycline Community-acquired: Minocycline TMP/SMX Clindamycin |
|
|
Community-Associated Methicillin-Resistant S. aureus (CA-MRSA)
|
Definition:
* MRSA specimen obtained outside hospital setting or within 48 hours after hospital admission * No clinical culture with MRSA in previous 6 months * None of the following within 1 year before infection: - Hospitalization - Admission to nursing home, SNF, or hospice - Surgery - Hemodialysis *Patient without permanent indwelling catheters or medical devices CA-MRSA characterized by: * Presence of SCCmec type IV - Mobile DNA cassette containing the gene that encodes for methicillin resistance (mecA) and other genes necessary for integration into the bacterial chromosome - SCCmec Type IV may be associated with more rapid replication, greater fitness than strains with other types * Most HA-MRSA strains possess SCCmec Type II * Presence of gene encoding Panton-Valentine Leukocidin (PVL) toxin, an important virulence factor * Lack of plasmids encoding for multidrug resistance, as is typical of hospital–acquired strains |
|
|
Can Community-Associated MRSA (CA-MRSA) cause Healthcare-Associated Infections
|
Yes,
* MRSA clinical isolates from 37 VA patients characterized according to molecular and epidemiologic types * Among patients classified with healthcare-associated MRSA infections, 60% were infected with strains with markers for CA-MRSA - SCCmec type IV genes - USA300-type strains by PFGE - + for Panton-Valentine Leukocidin (PVL) toxin genes * Patients with healthcare-associated bacteremia were as likely to be infected with CA-MRSA strains as patients with community-acquired infection (P = 0.38) * Second study also found CA-MRSA strains in 56% of healthcare-associated infections |
|
|
How is Clindamycin resistance inducible in Community-Associated MRSA?
|
* Primary mechanism of clinically relevant clindamycin resistance is ribosomal methylation
- Strains capable of expressing erm gene may be either constitutive (MLSBc) or inducible (MLSBi) phenotypes * Approximately 30-50% of CA-MRSA strains possess MLSBi * Although clinical data are limited, clindamycin treatment failures appear to be more likely in MRSA infections due to MLSBi strains - More likely in deep-seated, more severe infections * Although MLSBi resistance is not readily detected by standard in vitro testing methods, use of the “D-zone test” is simple and inexpensive |
|
|
Discuss the “D test”
|
* MRSA isolates should be routinely tested for MLSBi using the D-zone test, especially in areas where CA-MRSA is common
* A MRSA sample is isolated and inoculated on a petri dish to which antibiotic disks are added; Erythromycin and Clindamycin - The bacteria grows freely right up to the Erythromycin disk - The bacteria has a wide zone of inhibition around a clindamycin disk far from the Erythromycin disk - A blunted zone of inhibition is evident on the side of the clyndamycin near the erythromycin disk, creating a D looking pattern - This indicates a positive D-test meaning that clindamycin resistance was induced - Do not treat with Clindamycin |
|
|
Recommendations for Use of Clindamycin in CA-MRSA Infections
|
* MRSA isolates should be routinely tested for MLSBi using the D-zone test, especially in areas where CA-MRSA is common
* Non-MLSBi strains: clindamycin may be safely used * MLSBi strains: - Appropriate use of clindamycin is not clear; close follow-up and monitoring is needed if used - Clindamycin should not be used for more severe infections or those associated with high bacterial burdens * More complicated skin/soft tissue infections, i.e., abscesses, extensive tissue involvement * Endocarditis * Osteomyelitis |
|
|
What is the skinny on hVISA?
|
We don't know....
* Most clinical laboratories do not routinely, or correctly, screen for hVISA * True prevalence of hVISA is unknown * Clinical relevance of hVISA is unknown - Are patient outcomes worse when infected with hVISA strains of MRSA? * Optimal therapy of hVISA is unknown |
|
|
What are the prefered agents to use against PCN Susceptible Streptococcus pneumoniae?
|
PCN G or PCN V
Ampicillin/amoxicillin 1st gen. cephs “True" 2nd generation cephs Azithromycin Clarithromycin Doxycycline |
|
|
What are the prefered agents to use against PCN Intermediate S. pneumoniae?
|
Ceftriaxone
Cefotaxime Levofloxacin Moxifloxacin Gemifloxacin Vancomycin Azithromycin Clarithromycin Telithromycin |
|
|
What are the prefered agents to use against PCN Resistant S. pneumoniae?
|
Levofloxacin
Moxifloxacin Gemifloxacin Telithromycin Vancomycin Quinupristin/dalfopristin Linezolid Daptomycin Telavancin Tigecycline |
|
|
What are the prefered agents to use against Vancomycin Susceptible Enterococcus spp.?
|
Penicillin
Ampicillin Vancomycin Linezolid Quinupristin/dalfopristin Daptomycin Chloramphenicol Tigecycline Nitrofurantoin |
|
|
What are the prefered agents to use against Vancomycin Resistant Enterococcus spp.?
|
Linezolid
Quinupristin/dalfopristin Daptomycin Chloramphenicol Tigecycline Telavancin? |
|
|
What are the prefered agents to use against Enterobacteriaceae?
|
Amoxicillin (esp. community-acquired)
Ampicillin (esp. community-acquired) Amoxicillin/clavulanate Piperacillin Ampicillin/sulbactam Ticarcillin/clavulanate Piperacillin/tazobactam 2nd, 3rd, 4th generation cephs (all) Carbapenems (all) Aztreonam Aminoglycosides (all) Fluoroquinolones (all) TMP/SMX Tigecycline Nitrofurantoin |
|
|
Why are Amoxicillin and Ampicillin prefered agents against community-acquired Enterobacteriaceae?
|
Because Nosocomial strains have increased beta-lactamase production and therefore resistance to PCNs. Community acquired strains do not.
|
|
|
How is the list of prefered or effective agents for use against Enterobacteriaceae altered by ESBLs?
|
The only agents remaining are:
Carbapenems (all) TMP/SMX Tigecycline +/- Nitrofurantoin |
|
|
Agents useful against Pseudomonas aeruginosa
***Must know this list*** |
Piperacillin
Piperacillin/tazobactam Ticarcillin/clavulanate Ceftazidime Cefepime Imipenem Meropenem Doripenem Aztreonam Ciprofloxacin Levofloxacin Aminoglycosides (all) |
|
|
Agents useful against Haemophilus influenzae and Moraxella catarrhalis
|
* Usually susceptible to:
- amoxicillin/clavulanate - TMP/SMX - tetracyclines - cephalosporins (esp: 2nd, 3rd, and 4th gen.) - macrolides - FQ * Often resistant to PCN, ampicillin, amoxicillin - β-lactamase production in approx. 25-40% of H. influenzae isolates - β-lactamases produced by >85-90% of M. catarrhalis |
|
|
Agents useful against Anaerobic Infections
|
Metronidazole
Clindamycin Penicillin (0ropharyngeal anaerobes only) Carbapenems (all) Ticarcillin/clavulanate Piperacillin/tazobactam Ampicillin/sulbactam Amoxicillin/clavulanate Tigecycline Cefoxitin Cefotetan Cefmetazole Chloramphenicol |
|
|
Summary of Differences between HA-MRSA and CA-MRSA
|
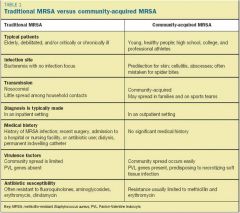
|

