![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
28 Cards in this Set
- Front
- Back
|
Name the dysfunctional ion channel in cystic fibrosis |
CFTR (cystic fibrosis transmembrane conductance regulator) |
|
|
What is the most common mutation that results in cystic fibrosis? |
DeltaF508 |
|
|
What does Delta F 508 mean? |
A single base pair deletion of phenylalanine at the 508th position |
|
|
What is a class I CFTR mutation? |
Protein is not produced at all. Due to insertions, deletions or substitutions that result in a shift in the reading frame |
|
|
What is a class II CFTR mutation? |
Protein is produced , but undergoes trafficking defects e.g. deltaF508 where the protein is destroyed before it reaches the membrane |
|
|
What is a class III CFTR mutation?
|
Protein is produced and inserted into the cell membrane, however does not respond to the correct signals |
|
|
What is a class IV CFTR mutation? |
Protein is produced and inserted into the cell membrane, responds to the correct signals but the channel component is altered so Cl ions cannot efficiently pass |
|
|
Describe the layout of an essay describing the inflammatory process in CF |
1. Discuss issue with CFTR, leading to inability to regulate ENaC. This leads to Na entering cells, water follows (in detail with diagrams, how salt is normally regulated) 2. This results in hyperplasia or goblet cells and sub-mucosal glands, and due to low amount of water there is a thick amount of dehydrated mucous 3. This creates an anaerobic environment which is preferred by usually harmless Pseudomonas bacteria 4. Pseudomonas are gram-negative bacteria that contain LPS, which is recognised by its PAMP via the TOLL-like receptor 4 on macrophages which leads to release of pro-inflammatory mediators that recruit neutrophils (e.g. TNF-alpha, IL-8 which recruit neutrophils) In a CF lung, there is no presence of IL-10 which usually down regulates neutrophils 5. Neutrophils attempt to phagocytose the Pseudomonas bacteria, but fail, and release its contents in a process known as 'frustrated phagocytosis' leading to release of neutrophil contents into the lungs (e.g. proteases and oxidant species such as H2O2, HOCl) 6. Introduction of proteases cause tissue damage, they are usually degraded by anti-proteases e.g. alpha-1 antitrypsin, however as there is less of these than the shear amount of proteases, tissue damage occurs. Oxidants normally broken down by fat soluble vitamins e.g. vitamin E, vitamin A and vitamin C, which are all at decreased levels due to pancreatic duct damage 7. Neutrophil elastase: Neutrophil Elastase is CENTRAL to the lung damage and repeatedbacterial infection in the lungs. It is one massive nasty vicious cycle ofinfection and repeated hyperinflammation ● It causes breakdown of C3b and IgG (which are main opsonins) preventingopsonisation of bacteria and therefore bacterial infection occurs again ● The bacterial infection causes an increase in neutrophils and IL-8concentration (causing more neutrophil production, which also increaseIL-8 production) the neutrophils also release DNA which plug the airwaysalong with mucous ● The increase in neutrophil concentration causes release of more neutrophilelastase via frustrated phagocytosis ● Elastase increases epithelial IL-8 concentration forming anotherinflammation cycle ● Elastase increases mucous secretion causing plugging of airways ● Elastase also obviously degrades the elastin in the lungs leading tobronchiectasis ● Neutrophils die via necrosis (untidy cell death) releasing actin whichthickens the mucous |
|
|
Describe the structure of CFTR
|
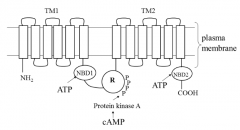
Contains two transmembrane domains (TM1 and TM2) each domain has six membrane spanning regions each, between the domains is where chloride ions enter. The loops shown are the result of glycosylation
Contains two nucleotide binding domains (NBD1 and NBD2) Contains one regulatory domain |
|
|
Describe how the chloride ion channel can be opened and closed (CFTR) |
Two ways in which it can be opened: 1. Phosphorylation of NBD1 (aid of ATP) this is deactivated by phosphorylation of NBD2 2. Direct phosphorylation of regulatory protein, this is deactivated by dephosphorylation of regulatory domain |
|
|
Describe the macromolecular complex assembly that occurs upon binding of beta-2 agonists to beta-adrenoceptors (with CFTR) |
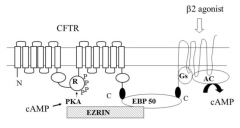
Beta-2 agonist e.g. salbutamol binds to 7TM spanning (G) protein, which activates adenelyl cyclase causing an increase in cAMP concentration The G-protein is linked to Ezrin Binding Protein 50 (EBP50) which forms a trimolecular complex with CFTR-EBP50-G-Protein. This is to ensure that the increase in cAMP is highly local and compartmentalised to cause an increase in phosphorylation of the regulatory domain Ezrin is a cytoskeletal protein, it is bound to Protein kinase A, it ensures that the cAMP reaches the regulatory protein easily |
|
|
CFTR protein undergoes post translational modification, specifically..... |
Glycosylation |
|
|
Describe how cystic fibrosis is a multi-organ disease (including examples): |
LUNGS: Most dangerous effects Repeated cycles of hyperinflammation and infection result in most deaths inflammation is unique to the lung in this disease SWEAT GLANDS: Chloride ions usually reabsorbed (CFTR open) and this affects sodium reabsorption and leads to salty sweat PANCREAS: Pancreatic exocrine duct blocking due to defective chloride secretion means that many important digestive enzymes are not released and malnutrition is therefore common State the diagnostic triad and how it's used to diagnose CF:The lack of lipase enzymes means it is difficult to absorb fat soluble antioxidant vitamins e.g. Vitamin A, D and E |
|
|
State the diagnostic triad and how it's used to diagnose CF: |
1. Excessively salty sweat (sweat gland defect) 2. Failure to thrive and gain weight (pancreatic defect) Persistent chest infection (as defective chloride secretion in lungs means that ENaC cannot be negatively regulated, leading to movement of sodium from surface of epithelium into epithelial cells, water follows leading to thick dehydrated mucous, setting scene for infection) |
|
|
With the aid of a diagram, describe salt regulation in a normal persons lungs
|
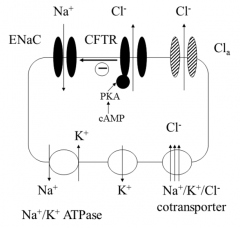
Normally, chloride ions move into the cell via the Na+/K+/Cl- transporter in the basolateral membrane CFTR is activated by phosphorylation of the regulatory domain or by hydrolysis of ATP bound to NBD1, this causes chloride ions to exit the cell via the apical membrane into the mucus layer (although chloride can also leave via other chloride channels, but most via CFTR) CFTR negatively regulates ENaC therefore sodium uptake is limited (and therefore water reabsorption) Water tends to follow the chloride ions, hydrating the mucous allowing the fluid cilia layer to waft it up The Na+/K+ ATPase in the basolateral membrane maintains the low intracellular Na+ concentration therefore water is usually moving into the airways |
|
|
With the aid of a diagram, describe salt regulation in a CF patients lungs |
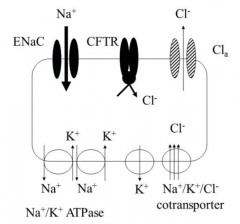
● CFTR is either absent or nonfunctional ● Therefore there is no negative regulation of the ENaC, and there is around10x more sodium reabsorption into the epithelial cell ● This increased intracellular sodium concentration is compensated by anupregulation of Na+/K+ ATPases. This means more ATP needs to beproduced by aerobic respiration and therefore more oxygen is used up ● The increase in sodium is followed by water, which dehydrates thesecretions making the mucous thick and sticky ● Mucous is produced in the submucosal glands (which are hyperplastic andsecrete a lot more mucous than they should) and goblet cells ● So a lot of thick and sticky mucus that is dehydrated is produced, thisleads to neutrophilic inflammation (as shown below) |
|
|
Why is IL-10 not present in a CF lung? |
Could be due to genetic defect or increased proteosome levels |
|
|
Describe how neutrophil elastase has its effect on the lungs (with a diagram) MUST GET EVERY ONE for correct answer |
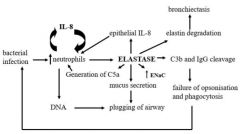
Neutrophil Elastase is CENTRAL to the lung damage and repeatedbacterial infection in the lungs. It is one massive nasty vicious cycle ofinfection and repeated hyperinflammation ● It causes breakdown of C3b and IgG (which are main opsonins) preventingopsonisation of bacteria and therefore bacterial infection occurs again ● The bacterial infection causes an increase in neutrophils and IL-8concentration (causing more neutrophil production, which also increaseIL-8 production) the neutrophils also release DNA which plug the airwaysalong with mucous ● The increase in neutrophil concentration causes release of more neutrophilelastase via frustrated phagocytosis ● Elastase increases epithelial IL-8 concentration forming anotherinflammation cycle ● Elastase increases mucous secretion causing plugging of airways ● Elastase also obviously degrades the elastin in the lungs leading tobronchiectasis ● Neutrophils die via necrosis (untidy cell death) releasing actin whichthickens the mucous - Increase ENaC's further contributing to thickening of mucous |
|
|
State major therapeutics used in cystic fibrosis, with details of mechanisms where appropriate |
Mucolytics: Break down the thick mucous to provide symptomatic relief (not usually by breaking down mucin like COPD, but breaking down the DNA elements from neutrophil necrosis). The only mucolytic with proven efficacy in CF is Dornase alfa (think door, nasal, alpha romao) Antibiotics: Wide range NSAIDs: Can control neutrophillic inflammation Anti-proteases: e.g. alpha-1 antitrypsan via inhalation are being tested Gene therapy: Realistically difficult Disease modifying agents: VX809 (lumacaftor): Corrects misfolding, hence fixing the root cause for patients with class 2 mutations e.g. deta F 508h (increases the number of CFTR channels moved into the cellmembrane, rather than degraded by proteaosomes Kalydeco: Potentiates Cl- ion conductance in patients with the G551D |
|
|
The only mucolytic with proven efficacy in cystic fibrosis is |
Dornase alfa |
|
|
State the names of the the two disease modifying agents used in cystic fibrosis treatment |
Lumacraftor (corrects misfolding hence places more channels in membrane in patients with deltaF508 mutations) Kalvdeco (potentiates chloride ion conductance in patients with G551D mutation) |
|
|
What is the mechanism of Kalvdeco
|
G551D is a single base pair substitution of a glycine for aspartic acid at the551st position, this only affects the conductance of the protein (usuallyplaced in the membrane) so a potentiator (kalydeco) is used to overcome this |
|
|
What is the mechanism of lumacraftor |
Acts as a pharmacological chaperone in the golgibody which increases the number of CFTR channels moved into the cellmembrane (corrects the misfolding) it is given in combination with Ivacaftor(orkambi brand name, given for deltaF508) which potentiate the protein,aiding the opening of the chloride ion channel |
|
|
Briefly describe the structure and function of the skin (different layers and functions of the relative layers) and also state and describe the function of the four major cell types found within the epidermal layer. State the four main layers of the epidermal layer of skin (including 5th in thick skin) |
Epidermis: Formed of epithelial tissues (no blood vessels) Dermis: Formed papillary and reticular region of connective tissue (collagen and elastin) contains the apocrine (body odour) and eccrine sweat glands (watery sweat for thermoregulation) phagocytic macrophages also found in this layer Subcutaneous layer for insulation and shock absorption Keratinocytes - Produce keratin (90% of all cells) and are stratified to protect skin, also produce laminar granules that release antimicrobial lipids that reduce water loss e.g. ceramide and sphingosine Melanocytes - Release melanin (8% of all cells) Langerhans cell - APC of the skin Merkel cell - Contact sensory neurons, detect touch Stratum corneum: 25-30 layers of dead corneocytes, form a CORNIFIED cell envelope involving the protein FILAGGRIN which aggregates the keratin cytoskeleton, allowing collapse into outermost layer of cells. Filaggrin is also important for hydration of the epidermal layer (corn is on surface) Stratum lucidum: Also dead cells, only present in hard skin Stratum granulosum: Consists of keratinocytes undergoing apoptosis(eventually be part of stratum lucidum and stratum corneum) releasing keratin and laminar granules which release the hydrating and antimicrobial lipids Stratum spinosum: Langerhans cells and melanocytes found here, spiny projections tightly link cells together Stratum basalis: Cuboidal or columnar keratinocytes |
|
|
Describe the skins innate defenses against infection |
Tight junctions between epithelial cells provide a barrier to infection (interlocking keratinocytes) Cornified outer layer of skin (keratin and filaggrin)Antimicrobial lipids (e.g. ceramide and sphingosine) and antimicrobial peptides (certain defensins e.g. LL-37)Acidic pH of sweat and defensins in sweat |
|
|
Define atopy and describe what is meant by the atopic march
|
Atopy: An inherited state of susceptibility to hypersensitivity reactions usually due to high levels of IgE. This is present in 80% of patients (with AD) and they are more likely to suffer from other hypersensitivity reactions e.g. rhinitis and allergic asthma Atopic march: The idea that barrier dysfunction (issues with the cornified stratum corneum layer) that occurs in AD, can lead to downstream systemic allergies via sensitisation of allergens through the skin e.g. allergic rhinitis, asthma and food allergies. The sensitisation occurs due to the epicutaneous damage, allowing allergens to enter, be processed and presented by Langerhans cells and lead to Th2 cell proliferation after entering the lymph nodes, which leads to systemic allergies. |
|
|
Describe symptoms, aetiology and pathophysiology of Atopic dermatitis
|
Symptoms: - Itchy, dry and red skin usually in folds of the armms, back of knees, wrist face and hands. This is worsened by the itch scratch cycle. - Characteristic lichenification (hypertrophy of epidermis) and papules (small raised bumps) are observed Aetiology: - Atopy - Fillaggrin defect (inherited or acquired) - Low levels of antimicrobrial lipids (ceramide and sphingosine)/antimicrobial peptides (LL-37) - Defects in innate immunity Pathophysiology: 1. Fillaggrin defect: an epidermal barrier defect Filaggrin is required to maintain hydration of the epidermis (stratum corneum) keeps the water in the body. It also contributes to the cornified cell envelope. Therefore it's a moisturizing factor. Lack of filaggrin therefore leads to transepidermal water loss (TEWL) leading to dry and flaky skin which is very itchy. The severity of the AD has been shown to be proportional to the extent of TEWL (caused by fillagrin defect) Can be inherited (defect in filaggrin gene) or aquired (Overproduction of IL-13 and IL-4 from Th2 cells and release of sphingosylphosphorylcholine from normal human keratinocytes, both act to down regulate the filaggrin gene) 2. Decreased antimicrobial peptides an epidermal barrier defect Overproduction of Th2 cell cytokines by dermal lymphocytes e.g. IL-4 and IL-13 can lead to decreased production of antimicrobial peptides (AMPs) e.g. LL-37 from keratinocytes 3. Decreased antimicrobial lipids (ceramide and sphingosine) an epidermal barrier defect Stress (itch) induced glucocorticoid release e.g. endogenous cortisol reduces the levels of ceramide and sphingosine which are required to maintain hydration in the epidermis and prevent infection. 4. Innate immunity defect: AD keratinocytes have a defective response to microbes - Cortisol released due to physiologic stress decreases keratinocyte production of IL-1beta (robust innate immunity, neutrophil recruitment) and IL-18 (which is involved in induction of IFN-gamma and Th1 responses). This exacerbates AD by increasing infection - PMN defect (defective chemotaxis, phagocytosis and superoxide generation) and also defect in PMN chemoattractants (PAF receptors, formylated protein receptors) - Polymorphisms in TLR2 and CD14 which recognise bacterial endotoxins and cell wall components. - High [IL-1R antagonists] means a dampened IL-1 mediated immune responses - Characterised by Th2 cells release a lot of IL-4 and IL-13 , which lead to the following: -Reduction in antimicrobial peptide (e.g. LL-37) formation - Increase in IgE isotype of antibodies (via isotype switching) - Upregulation of Fc-epsilon R1 therefore more mast cells and eosinophils with IgE bound - -- -- - Decreased levels of filaggrin, lack of filaggrin therefore leads to transepidermal water loss (TEWL) leading to dry and flaky skin which is very itchy (there is a very clear correlation between AD severity and amount of TEWL) 5. SA mediated tissue damage: - Staphlococcus aureus is present in around 90% of patients with AD - Contains superantigens, which are able to cross link MHC-II without processing and presentation on MHC-II - Very potent and stimulate proliferation and cytokine production from T-cells (this excessive production of IL-2, INF-gamma and TNF-alpha can lead to uncontrolled inflammation and further tissue damage - Also leads to production of TSLP (thymic stromal lymphopoietin) which up regulates the T-cell populations leading to further inflammation. (the role of this not completely known.) |
|
|
Describe treatment options for atopic dermatitis |
Ceramide based moisturizers: e.g. epiceram (replenish the ceramide, reducing barrier defect)Antiseptics and antibiotics for SA infection: e.g. chlorhexidine and neomycin respectively Topical corticosteroids to reduce Th2 mediated inflammation: e.g. hydrocortisone, usually not applied to face as it causes thinning of the skin, and the face already has thin skin, also prevent SA binding to the skin Calcineurin inhibitors: e.g. tacrolimus or cyclosporin inhibit T-cell proliferation by preventing activation of NFAT by inhibition of calcineurin, reducing T-cell potency, same issues as all steroid creams Mycophenolate: Non-competitive, selective and reversible inhibitor of inosine-5’-monophosphate dehydrogenase (IMPDH), which is a key enzyme in de-novo guanosine nucleotide synthesis. Unlike other human cells, B and T-cells are very dependent on this process and mycophenolate inhibits their production Methotrexate: Reversible inhibitor of dihydrofolate reductase, prevents T-cell proliferation |

