![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
56 Cards in this Set
- Front
- Back
|
What is the structure of Strep. pyogenes?
|
>Gram positive cocci in chains
>Thick peptidoglycan wall with M proteins >Hyaluronic acid capsule |
|
|
How is Streptococcus classified?
|
>By reactions in various culture media
- β haemolytic on blood agar >Lancefield classification - Antigenicity of cell wall carbohydrate - Group A |
|
|
How does Streptococcus differ from gram negative organisms?
|
>It possesses a peptidoglycan cell wall (murein) which stains dark purple with gram stain.
|
|
|
Where is Strep. pyogenes found in the body?
|
>Skin
>Throat >Oro-nasopharynx NB. Attaches via M protein |
|
|
How is Strep. pyogenes transmitted?
|
>Respiratory droplets (aerosol)
|
|
|
How does the host respond to Strep. pyogenes?
|
>Acute inflammatory response
- Endogenous and exogenous mediators (IL8 and PG) - Activation of macrophages and mast cells - Complement activation - Humoral immunity (opsonisation) |
|
|
What virulence characteristics of Strep. pyogenes contribute to avoiding the host defence mechanisms?
|
SUPPURATIVE INFECTION:
>Hyaluronidase: mediates subcut. spread (cellulitis) >Erythrogenic toxin (superantigen) causes scarlet fever rash >M protein impedes phagocytosis IMMUNOLOGIC REACTIONS >Immunologic cross reaction between antigen and human and joint tissue - Rheumatic fever - anti-M antibody + myosin in the heart >Immune complexes between strep antigens and antibody to these become trapped in glomeruli, activating complement and taxing neutrophils which release damaging proteases |
|
|
What products of Strep. pyogenes infection are responsible for tissue damage?
|
Pathogen-associated molecular patterns (PAMPs)
>Hyaluronidase >Bacterial antigen >M protein |
|
|
How does Strep. pyogenes infection manifest itself?
|
SUPPURATIVE:
>Pharyngitis - reddened pharynx with exudates generally present; cervical lymphadenopathy >Scarlet fever - diffuse erythematous rash beginning on the chest and spreading to the extremities >Pyoderma - localised skin infection with vesicles progressing to pustules >Erysipelas - localised skin infection with pain, inflammation, lymph node enlargement and systemic symptoms >Cellulitis - infection of the skin involving subcut. tissues >Necrotising fascitis - deep skin infection involving destruction of muscle and fat layers >Strep. toxic shock - multiorgan systemic infection resembling staphylococcal toxic shock syndrome NON SUPPURATIVE: >Rheumatic fever - Inflammatory changes of the heart (pancarditis), joints (arthralgias to arthritis), blood vessels and subcut. tissues >Acute glomerulonephritis - acute inflammation of the renal glomeruli with oedema, hypertension, haematuria and proteinuria. |
|
|
How is Strep. pyogenes treated, and prevented?
|
>Penicillin G/Benzylpenicillin
- can be used prophylactically |
|
|
What is the structure of Strep. pyogenes?
|
>Gram positive cocci in chains
>Thick peptidoglycan wall with M proteins >Hyaluronic acid capsule |
|
|
How is Streptococcus classified?
|
>By reactions in various culture media
- β haemolytic on blood agar >Lancefield classification - Antigenicity of cell wall carbohydrate - Group A |
|
|
How does Streptococcus differ from gram negative organisms?
|
>It possesses a peptidoglycan cell wall (murein) which stains dark purple with gram stain.
|
|
|
Where is Strep. pyogenes found in the body?
|
>Skin
>Throat >Oro-nasopharynx NB. Attaches via M protein |
|
|
How is Strep. pyogenes transmitted?
|
>Respiratory droplets (aerosol)
|
|
|
How does the host respond to Strep. pyogenes?
|
>Acute inflammatory response
- Endogenous and exogenous mediators (IL8 and PG) - Activation of macrophages and mast cells - Complement activation - Humoral immunity (opsonisation) |
|
|
What virulence characteristics of Strep. pyogenes contribute to avoiding the host defence mechanisms?
|
SUPPURATIVE INFECTION:
>Hyaluronidase: mediates subcut. spread (cellulitis) >Erythrogenic toxin (superantigen) causes scarlet fever rash >M protein impedes phagocytosis IMMUNOLOGIC REACTIONS >Immunologic cross reaction between antigen and human and joint tissue - Rheumatic fever - anti-M antibody + myosin in the heart >Immune complexes between strep antigens and antibody to these become trapped in glomeruli, activating complement and taxing neutrophils which release damaging proteases |
|
|
What products of Strep. pyogenes infection are responsible for tissue damage?
|
Pathogen-associated molecular patterns (PAMPs)
>Hyaluronidase >Bacterial antigen >M protein |
|
|
How does Strep. pyogenes infection manifest itself?
|
SUPPURATIVE:
>Pharyngitis - reddened pharynx with exudates generally present; cervical lymphadenopathy >Scarlet fever - diffuse erythematous rash beginning on the chest and spreading to the extremities >Pyoderma - localised skin infection with vesicles progressing to pustules >Erysipelas - localised skin infection with pain, inflammation, lymph node enlargement and systemic symptoms >Cellulitis - infection of the skin involving subcut. tissues >Necrotising fascitis - deep skin infection involving destruction of muscle and fat layers >Strep. toxic shock - multiorgan systemic infection resembling staphylococcal toxic shock syndrome NON SUPPURATIVE: >Rheumatic fever - Inflammatory changes of the heart (pancarditis), joints (arthralgias to arthritis), blood vessels and subcut. tissues >Acute glomerulonephritis - acute inflammation of the renal glomeruli with oedema, hypertension, haematuria and proteinuria. |
|
|
How is Strep. pyogenes treated, and prevented?
|
>Penicillin G/Benzylpenicillin
- can be used prophylactically >Clindamycin is used in the treatment of necrotising fascitis |
|
|
How is strep. Pyogenes diagnosed?
|
SUPPURATIVE:
>Gram stained smear and culture - β-haemolytic colonies on blood agar - Sensitive to bacitracin >Rapid ELISA available for S. Pyogenes (QUERY THIS) NON SUPPURATIVE: >Antistreptolysin O (ASO) antibody titre for suspected Rheumatic fever) >Antibody to strep DNAase B used as evidence for acute glomerulonephritis |
|
|
Describe the structure of Mycobacterium Tuberculosis.
|
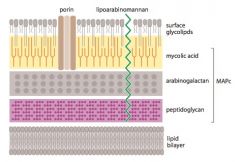
>Weakly gram +ve bacillus; classified as 'acid-fast' because Ziehl Neelsen stain does not come off after washing with acid
>Contains: - Mycolatearabinogalactan-peptidoglycan complex MAPc - Mycolic acid layer impervious hence porins on surface to allow hydrophilic compounds through - Mycolate plays major role in defence of cell because not penetrated by most Ab, resistant to infection and some disinfectants NB. Lipoarabinomannan and mycolic acid are main virulence factors |
|
|
What are the physiological characteristics of mycobacteria?
|
>Mycobacterium can be broadly divided into rapid and slow growers and non-cultivable species:
- Mycobacterium leprae (causes leprosy; cultivable in armadillos only) - Mycobacterium smegmatis; Lowenstein Jensen, 2-3 days - Mycobacterium TB; Lowenstein Jensen; 2-4 weeks culture time >Slow growth raises problems in determining illness causing species |
|
|
How is M. Tuberculosis spread?
|
>M. Tuberculosis bacilli from granulomas are released into the bronchi and are spread through coughing
- Aerosols contain droplet nuclei and survive for long periods outside the body - Each person infects approximately 20 others - Repeated contact with an infected individual (particularly in a closed environment, produces higher transmission rates than casual contact >Smear positive patients indicates bacteria present in large numbers in airways :. more contagious - 50% contacts infected - Smear neg. 5% of contacts infected >Number of coughs directly related to transmission rate |
|
|
What is the global prevalence of Tuberculosis?
|
>Approx. 1/3 of global population infected
>8-10M annual incidence rate >2M mortality rate (annual) >New person infected every second - forecast 2020 mortality could be 4M... >TB responsible for 1/4 preventable deaths |
|
|
How does the host respond to M. Tuberculosis?
|
INNATE: MACROPHAGE MEDIATED
>MØ phagocytose M. TB which live and divide within the MØ - Inhibiting maturation of phagosome (cord factor and trephalose dimycolate) - Causing autophagy (lysosomal fusion with organelles) >MØ activation: - Cytokine production: initiating the inflammatory response recruiting further MØ - Overriding the M. TB mediated block of phagosome maturation; - Upregulation of antimicrobial effectors (NADH oxidase which generates reactive oxygen and nitrogen species) ADAPTIVE: T-CELL MEDIATED >Some fragments are presented via HLA II to CD4+ T cells - MØ and IDC IL-12 / IL-18 induces TH1 cell production - Th1 cells produce pro-inflammatory cytokines IFN-γ and TNF-α; along with MØ IL-1 and IFN-γ activates defensins e.g. cathelicidin, NO and stimulates autophagy >CD8+ T cells are induced through HLA I - Produce IFN-γ - also kill TB infected cells using perforin and granzymes (granulysin) CHRONIC INFECTION: >TB persists in most cases: - Continuous activation of both CD4+ and CD8+ cells - Cytokines released by these and MØ lead to the development of granulomas (tubercles) limiting disease spread - become surrounded by connective tissue layer walling it off from the rest of the body (containment or latent phase) |
|
|
What virulence characteristics of M. Tuberculosis contribute to avoiding the host defence mechanisms?
|
>Mycolic acid
>Lipoarabinomannan |
|
|
What products are responsible for tissue damage in M. Tuberculosis infection?
|
>Products of host immune response:
- Granulysin (CTLs) - Cytokines released by CD4 and CD8 T cells / MØ (IFN-γ and TNF-α) >Granulomatous response = Type IV hypersensitivity reaction (classic chronic inflammation) |
|
|
What is the mechanism of tissue damage in M. Tuberculosis infection?
|
>Cytokine production leads to granuloma formation (collection of active and resting macrophages - epithelioid cells) surrounding necrotic tissue
>Local tissue destruction caused by host response - cytokines cause further damage >Granulomas may cavitate, spreading bacilli |
|
|
How is disease clinically manifested in M. Tuberculosis infection?
|
>Fever - TNF-α
>Chronic productive cough with haemoptysis (rupture of bronchial arteries) >Cachexia >Mediastinal lymph node swelling >Cavitation >Granulomatous spread - brain, bone, liver (Addison's, Pott's disease) |
|
|
How is M. Tuberculosis diagnosed in the laboratory?
|
>Gold standard - Lowenstein Jensen media
>Sputum smear - Ziehl Neelsen stain (50% sensitive) >Elispot test assaying T-cell responses to mycobacterial antigens >IFN-γ assay >PCR NB. Differential - lung tumour, silicosis, sarcoidosis |
|
|
What is the treatment of M. Tuberculosis infection? How may it be prevented?
|
>Isolation, -ve pressure
>Rifampicin and Isoniazid and Pyrizinamide +/- Ethambutol (6 months) >Levofloxacin considered for MDR TB >Vaccination (Bacillus-Calmette-Guerin) attenuated M. Bovis - 80% effective in Northern Europe. |
|
|
Describe the infection cycle of Mycobacterium Tuberculosis?
|
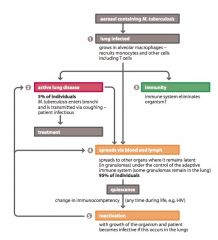
|
|
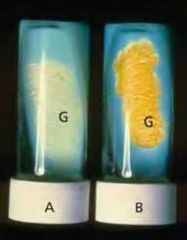
Identify this medium.
|
Lowenstein Jensen
|
|
|
What sort of organism is Plasmodium?
|
>Eukaryotic protozoan that infects erythrocytes
- Possesses eukaryotic organelles - Four species: 1. P. Falciparum (most virulent) 2. P. Ovale 3. P. Vivax 4. P. Malariae |
|
|
How is malaria spread?
|
>Transmission stage of P = sporozoite which is injected into the bloodstream of a human when the female Anopheles takes a blood meal.
>Following a bite, at least some of the sporozoites remain in the dermis before entering bloodstream and draining lymph nodes - Few dozen sporozoites transmitted during feeding but rapid translocation into the liver to begin first stages of disease SUMMARY: >Person-person via mosquito >Non vector - transfusion, hypodermic needle sharing, accidents, vertical transmission |
|
|
Where is malaria found and what is the global disease burden?
|
>Mostly sub-saharan Africa (80% of cases, 90% of all people carrying parasite)
- Asia, LatAm, Middle East and Europe - Increase in spread due to rise in international travel >Global incidence 350-500M cases per year |
|
|
How does the host defend itself against infection?
|
HUMORAL IMMUNITY:
>Ab produced against: - Initial entry of sporozoites into blood and lymph - Merozoites in bloodstream - Male and female gametocytes in bloodstream - Intraerythrocyte stage CELLULAR IMMUNITY: >Intra hepatocyte: - CD4+ T cells - CD8+ T cells >Cytokines / other factors - Reactive oxygen/nitrogen intermediates - Eosinophil chemotactic factor - TNF VECTOR IMMUNITY >Innate immunity thought to be induced in the mosquito to the gametes and sporozoites of Plasmodium |
|
|
Describe the lifecycle of Plasmodium.
|
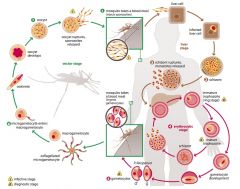
|
|
|
What are virulence factors of Plasmodium?
|
>Virulence factors include toxins and the surface adhesion molecule PfEMP-1
- a highly variant protein which enables immune evasion and parasite sequestration |
|
|
How does malaria parasite cause host damage?
|
INVASIVE DAMAGE:
>Invasion and destruction of liver tissues >Invasion and destruction of erythrocytes IMMUNOPATHOLOGY: >Mononuclear phagocytic system response and Hemozoin pigment accumulating in erythrocytes - stimulates release of cytokines from host cells e.g. TNF (pyrogen) >Destruction by infection and defective production of red cells (probably due to cytokines) leading to anaemia - Erythrocyte membrane modification causes inflexibility :. cells removed by spleen (splenomegaly) - Ab mediated lysis of erythrocytes occurs as parasite Ag expressed on erythrocytes >Capillary obstruction caused by parasitised erythrocytes (also due to membrane surface modifications) - Leads to cerebral malaria in Falciparum |
|
|
How does malaria present clinically?
|
INITIAL SYMPTOMS:
>Fever, headache, chills vomiting (10-15 days post infection) >Liver stage asymptomatic >Splenomegaly possible PROGRESSION: >Fevers become periodic depending on the parasite - Temperature fluctuations coincide with rupture of erythrocytes and release of merozoites into bloodstream, during erythrocytic stage - 48hrs for Falciparum, Ovale, Vivax - 72hrs for Malariae |
|
|
What are the complications of malaria?
|
FALCIPARUM:
>Cerebral complications, thrombosis due to occlusion of cerebral vessels caused by increase in erythrocytes >Multi-organ damage and renal failure >Blackwater fever due to intravascular haemolysis leading to haemoglobinuria and kidney failure >Pulmonary oedema >Possible splenic rupture MALARIAE: >Nephrotic syndrome |
|
|
How is malaria diagnosed?
|
>Blood film = mainstay
- Morphologic appearance of trophozoite or gametocyte can be used to identify the species >Serological diagnosis possible (direct or indirect ELISA) >PCR / DNA probe >Clinical diagnosis difficult because of overlap with other diseases |
|
|
How is malaria treated / prevented?
|
TREATMENT:
>Chloroquine, Quinine first line treatments (Falciparum building resistance to Chloroquine) >Artemisinin-based combination therapies: Mefloquine, Atavaquone, Proguanil, Tetracyclines used to treat Falciparum PREVENTION: >Vector control using nets or indoor spraying >Vaccines not available but are being developed - targeting erythrocyte, liver and sexual stage - prophylaxis available |
|
|
How is HIV diagnosed / confirmed?
|
>HIV diagnosis based on Ab tests either testing for Ab or Ag
- Rapid test kits available for HIV-1 (20 min for result) - Test blood plasma, serum, whole blood or saliva - Highly specific test with occasional false positives (although low prevalence in West) therefore test must be 'confirmed' to ensure correct diagnosis >HIV confirmation may carried out using: - Western Blot (USA) - Latex agglutination and ELISA - PCR >Necessary to take a second blood sample to ensure the correct blood is being tested NB. Ab tests aren't useful prior to seroconversion 'diagnostic window', so combination tests are used to test for IgG and p24 Ag - Necessary for neonates to directly test for viral nucleic acid (direct diagnosis) - Relative decrease of CD4+ cells in relation to CD8+ cells may also provide diagnostic evidence in babies |
|
|
By what route is HIV transmitted between individuals?
|
>HIV enters body via the MUCOSAL SURFACES or is INJECTED into the bloodstream via contaminated surfaces
>Principally transmitted through sexual intercourse (virus present in semen / vaginal fluid) - Globally heterosexual transmission dominates - Homosexual transmission dominates in the West >Transfer of blood via transfusion or contaminated needles is possible >Vertical transmission also occurs in utero, at birth or via breastfeeding |
|
|
How may HIV transmission be controlled / influenced?
|
>Testing, sex education, condom programmes and male circumcision have all been shown to impact transmission
>Effective ART also lowers viral load, slowing transmission Other initiatives: - Needle exchange programs for drug users - Careful and rigorous intra/inter hospital screening for HIV+ donations of human tissue - Neonatal support in developing countries (maternal education and neonatal PEP - PEP also offered as an option post-sexual exposure by some hospital trusts in the UK (offered as standard for occupational exposures e.g. needlestick injury |
|
|
What are the major classes of ART drugs in clinical use?
|
UK treatment guidelines:
>Recommendation to initiate HIV therapy once CD4+ count drops below 350 >Current therapies used: - PIs e.g. Atazanavir, Darunavir - NRTIs e.g. Emtricitabine, Tenofovir - NNRTIs e.g. Efavirenz, Etravirine - Fusion inhibitors e.g. Enfuvirtide - CCR5 inhibitors e.g. Maraviroc - IIs e.g. Raltegravir - HAART e.g. Efavirenz, Tenofovir, and Emtricitabine |
|
|
How is viral response to therapy monitored?
|
>Via viral load testing and CD4 count
- Viral load test via RNA PCR - <50 copies per ml considered undetectable - CD4+ count measured via flow cytometry to ensure patient not vulnerable to infection (500+ = low risk, <200 = high risk) NB. Viral load testing also used as a means to identify levels of resistance. |
|
|
What is meant by HIV resistance?
|
>Is a serious issue and has potential to enable spread of disease
- If patients are non-compliant with therapy this builds resistance - Also builds via reinfection with different strains of the disease, developing a supervirus >Individual testing and HIV therapy tailoring possible - Tested via viral load (RNA PCR) - Extremely expensive and only in developed countries |
|
|
Describe the clinical course of HIV infection.
|
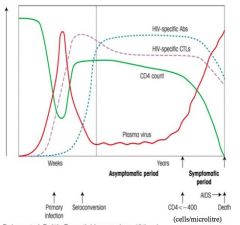
>HIV binds to and infects CD4+ Th cells where it replicates and gives rise to new visions by budding
- CD4 and chemokine co-receptor (CCR5 or CXCR4) are both required for binding and entry of HIV - Memory CD4 Th cells express mainly CCR5 and most CD4+ T cells in the lamina propria at mucosal surfaces are memory cells and are targeted by the virus - Dendritic cells are also important in the infection process and bind HIV via surface DC sign; - When dendritic cells present HIV or other Ag peptides to CD4+ cells the CD4+ cells become infected with HIV ACUTE PHASE: >Post primary infection, virus is carried by dendritic cells in the lamina propria to draining lymph nodes - Here virus production by CD4+ T cells leads to viraemia (7-14 days after infection) - Evidence shows increase in HIV specific CD8+ CTL responses which reduce viral load initially over 2-6m - Viraemia reaches a 'set-point' (individual) - Levels of CD4+ T cells decrease significantly but increase slightly as immune response begins to have some control over infection CLINICAL LATENCY: >Immune system is unable to clear the virus completely and a reservoir of latently infected CD4+ T cells and dendritic cells 'harbouring' the virus remains - during this clinically asymptomatic phase CD4+ T cells decrease and viraemia increases - Length of latency varies from person to person AIDS: When CD4+ count drops towards 200 cells / ml the patient may develop disease indicating onset of AIDS >Key event is death of CD4+ T cells, mechanisms for which are thought to include apoptosis: - directly mediated by virus - mediated by CD8+ cells recognising MHC I on the surface of infected CD4+ cells >Lymph nodes become sites of activated CD8+ T cells and B cells which eventually results in breakdown of follicular structure and fibrosis |
|
|
Describe the clinical presentation of HIV.
|
>Primary infection maybe asymptomatic
- 25-65% of patients may have some symptoms that usually begin about 2-8 weeks post exposure (and resolve rapidly) e.g. infectious mononucleosis-like illness with: - fever - maculopapular rash - sore throat - night sweats - malaise - lymphadenopathy - diarrhoea - mouth / genital ulcers - neurological disease - meningitis (neuropathy, myelopathy, encephalitis) >Clinical latency mostly asymptomatic: - 1/3 patients develop persistent generalised lymphadenopathy >CD4+ count below 200x10^6 / ml patients present with opportunistic infections and tumours (AIDS defining) - bacterial, fungal and viral infections - NHLs / KSs |
|
|
How does HIV kill cells / cause tissue damage?
|
1. Direct cell killing
- Large amounts of virus being produced and budding off from cell surface, disrupting membrane, or viral protein accumulation disrupting cellular machinery 2. Syncytia formation - Infected cells may fuse with nearby uninfected cells, through CD4+ mediated fusion, forming balloon-like giant cells called syncytia - Mechanism of cell-cell spread has been associated with the death of uninfected cells - Presence of syncytia forming variants has been correlated with rapid disease progression in HIV+ individuals 3. Apoptosis - Infected CD4+ T cells may be killed when cellular regulation is distorted by HIV proteins, leading to their suicide by programmed cell death - Uninfected cells may also undergo apoptosis - HIV envelop alone or bound to Ab sends an inappropriate signal to CD8+ cells causing them to undergo apoptosis even though they aren't infected by HIV 4. Innocent bystanders - Uninfected cells may die in an innocent bystander scenario - HIV particles may bind to the cell surface giving them the appearance of an infected cell and marking them for destruction by killer T cells 5. CNS damage - Infected macrophages and monocytes resist killing and subsequently travel throughout the body carrying HIV to various organs esp. lungs and brain - People infected by HIV often experience neurologic abnormalities, perhaps due to increased viral load or inappropriate cytokine release |
|
|
What is the nature and cause of Kaposi's Sarcoma?
|
>KS is a tumour of abnormal vascular structures
- Commonly found in skin, hard and soft palate and gums - diagnosis by clinical and histological appearance - no specific ARV to treat or vaccine - caused by HHV-8 >Now the most common neoplasm affecting HIV+ individuals |
|
|
What is IRIS and what is its cause?
|
>Immune Reconstitution Inflammatory Syndrome
- ARV action of strengthening immune response may over activate immune system - HAART potentially a cause |

