![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
122 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Describe the structure of the bacterial cell membrane (+ve and -ve).
|
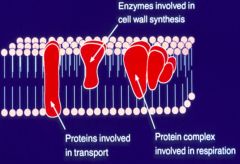
>Composed primarily of protein and phospholipid. It performs many functions, including transport, respiration biosynthesis of phospholipid, and energy transduction.
>Space between the plasma and outer membranes of Gram neg. bacteria is the 'Periplasmic space'. >Teichoic acids are negatively charged polymers found in Gram positive bacteria peptidoglycan - strongly antigenic. >Lipoteichoic acids are antigenic polymers anchored in the membrane. - Antigenic, cytotoxic and adhesins. >Lipopolysaccharides (LPS) are complex molecules consisting of a Lipid A anchor, a polysaccharide core, and a chain of carbohydrate molecules. - Present on the outer leaflet of the outer membrane of Gram-neg. bacteria. - Responsible for symptoms of gram negative sepsis (hence called endotoxin). |
|
|
|
What are bacterial endospores?
|
>E.g. Anthrax (Bacillus anthracis)
>These are developed by bacteria usually in response to nutrient deprivation. >Allows the bacterium to produce a dormant and highly resistant cell to preserve the cell·s genetic material in times of extreme stress. >Endospores can survive environmental assaults that would normally kill the bacterium. - These stresses include high temperature, high UV irradiation, desiccation, chemical damage and enzymatic destruction - Why C.Diff diarrhoea is so persistent in hospitals and care homes |
|
|
|
Describe the stages of a bacterial growth curve.
|
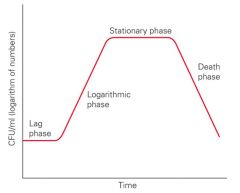
>During the lag phase the bacteria is adapting to its environment.
>Growth flattens out after rapid growth due to limitations of environment (otherwise would grow exponentially - bacteria grow by binary fission). >Eventually the bacteria enter the death phase. NB. Bacteria only produce enzymes if the substrate is present e.g. lac operon. They are more susceptible to attack when adapting to a new environment. |
|
|
|
Describe how and which bacteria metabolise O2.
|
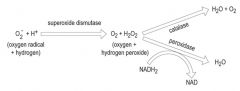
>Several commensal and pathogenic bacteria e.g. streptococci are unable to complete the final stages and secrete hydrogen peroxide.
Examples: >Strict aerobe: molecular O2 >Facultative aerobe: molecular O2 or an organic molecule >Strict anaerobe: organic molecule, NO3, SO4 >Microaerophiles - 2-10% >Capnophiles e.g. Strep pneumoniae - nasopharynx - ideal growth in increased CO2 5-10%. |
|
|
|
Describe how bacteria conduct respiration.
|
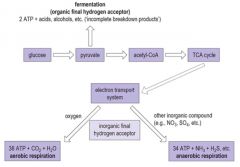
|
|
|
|
Describe the structure of the Gram +ve bacteria cell wall.
|
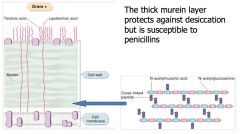
>Protective layer that determines the shape of bacterial cells.
>Contains superantigens (teichoic and lipoteichoic acids) - Can overstimulate the immune response causing sepsis and toxic shock. >Stains positive for Gram |
|
|
|
Describe the structure of gram -ve bacterial walls.
|
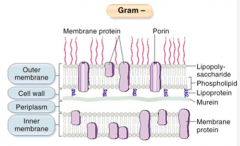
>Plasma membrane is covered with a thin layer of peptidoglycan cell wall which is then covered by a further outer membrane which may be covered by a polysaccharide capsule.
>Similar to Gram +ve bacteria, there are antigenic LPS (lipopolysaccharides) on the outer cell membrane which when broken down can act as a pro-inflammatory mediator - causing sepsis; also known as ENDOTOXIN. |
|
|
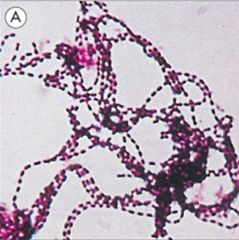
Identify this bacteria class.
|
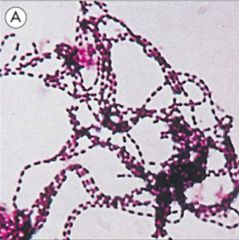
>Gram +ve
>Streptococcus >E.g. S. Pyogenes |
|
|
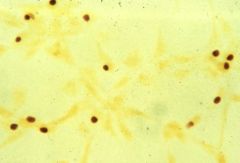
Identify this bacterial class and an example of its pathology in humans.
|
>Spirochaetes - comma shaped cells
>e.g. Campylobacter Jejuni: - common cause of diarrhoea - microaerophilic - require low oxygen tension and thermophilic (can grow at 42C) - Infection is via contaminated water milk or food - especially poultry. >Stimulated by organic farming; post infection association with Guillain Barre syndrome. |
|
|
What are these? Describe their characteristics.
|
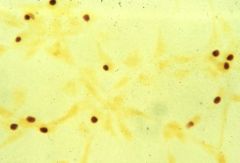
>Chlamydiae (0.2-0.8 μm)
>Obligate intracellular parasites with a Gram -ve cell wall >Unique developmental cycle with an infectious extracellular elementary body and a non-infectious intracellular reticulate body. >Cause eye and genital tract infections. |
|
|
|
What are flagella?
|
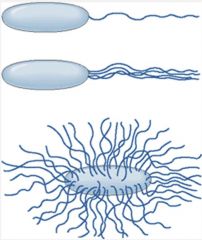
>Organ of locomotion, expressed by both Gram +ve and -ve bacilli but rarely cocci.
>Less than 12μm long (i.e. longer than the bacterium) and less than 30nm in diameter. >Made of proteins assembled into a long hollow cylinder anchored to the cell membranes via a hook and a basal body. >Rotates fast in helical twists which create movement towards nutrition (chemotaxis) or air (airotaxis) or away from noxious stimuli. >Amount and distribution of flagella are characteristic for any given species: - Polar or peritrichous |
|
|
|
What are bacterial pili?
|
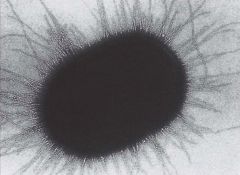
>Protein structures that are important in enabling pathogens to attach to surfaces.
>Play roles in the acquisition of external DNA and are required for twitching motility. >Hair like appendages of varying length and shorter than flagella. |
|
|
|
Describe the structure of the mycobacterium cell wall.
|
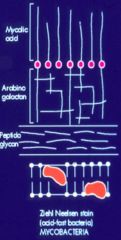
>Thick lipid rich cell wall which does not take gram stain
- Contain arabinogalactan and mycolic acids - Can be readily seen using a fluorochrome stain (needs UV microscope) >Mycobacterium include the pathogens that cause tuberculosis and leprosy. |
|
|
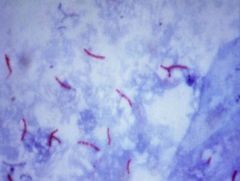
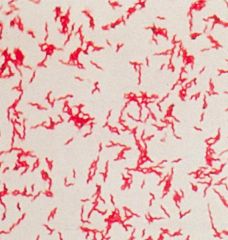
What type of stain is this?
|
>Ziehl-Neelsen
- Involves heat to allow stain to penetrate the cell wall - decolourises with acid (cold) and counter stains >Acid Fast Bacteria are visible (stain red), NAFBs stain green. |
|
|
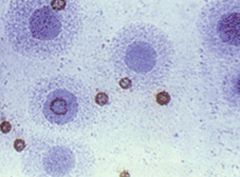
Identify this microorganism and describe its characteristics.
|
Mycoplasmas:
>The smallest organisms capable of growth on cell-free media >Lack a rigid cell wall >Require sterols for growth >Include four species that cause human disease: - Mycoplasma pneumoniae - M. genitalium - M. hominis - Ureaplasma urealyticum >"Fried egg" morphology of colonies >Causes bronchopneumonia |
|
|
|
Describe the structure of the fungal cell wall.
|
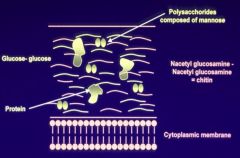
Made up of:
- Mannose - Glucosamine and chitin - Contain proteins |
|
|
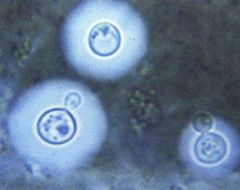
Identify this organism, and its staining and its associated pathology.
|
>Cryptococcus:
- India ink preparation (negative stain), showing capsules - Environmental yeast - Causes meningitis NB: Some fungi grow unicellularly, e.g. yeast, and multiply by budding or splitting; dimorphic fungi switch between yeast and mycelial forms. |
|
|
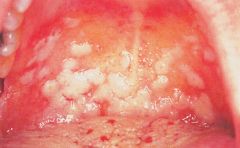
Identify this pathology.
|
>Oral thrush / milk tongue
>Caused by Candida spp. >Associated with antimicrobial therapy that disturbs the commensal bacterial microbiota and results in fungal overgrowth. |
|
|
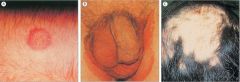
Identify these conditions.
|
RINGWORMS:
A. Tinea corporis - annular lesion spreading from centre B. Tinea cruris - jock itch C. Tinea capitis - scaling and hair loss on scalp NB. Athlete's foot another example. |
|
|
|
Describe the infection of Entamoeba histolytica.
|
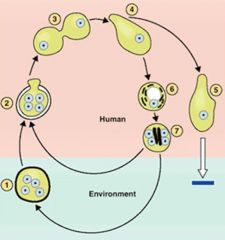
>Cysts are ingested and parasites emerge and multiply in the upper GI tract.
>Pathogenic trophozoites form in the colon and form cysts in unfavourable conditions. |
|
|
|
Describe the infection cycle of intestinal nematodes.
|
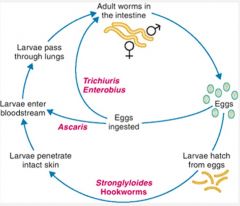
|
|
|
|
Describe what is meant by 'bacterial capsule'.
|
>Some bacteria form capsules which constitute the outermost layer of the bacterial cell and may be too think to be detected or up to 10μm thick.
>Bacterial capsules enable bacteria to survive inside the host and avoid phagocytosis. >Some organisms lack a well defined capsule but have loosely bound layers of polysaccharide-based slime which covers the organism. >Overproduction of slime may help to form biofilms. NB. Appear to have mucoid morphology. |
|
|
|
Describe the 5 key principles of ecological succession.
|
1. Transmission - spread
2. Acquisition - gain a foothold 3. Succession - divide and shed 4. Increase species diversity - vary colonisation 5. Climax community - reach the optimum ecosystem (finding a balance) |
|
|
|
Which ecological factors influence microbiota? (7)
|
>Attachment or retention at surface (Skin, upper respiratory tract, GU tract)
>Humidity (skin) >Levels of oxygen and CO2 >Nutrients >pH (mouth, stomach, vagina) e.g. helicobacter survive in a low pH >Antagonists - From host - From commensal microbiota e.g. collicins |
|
|
|
What particular characteristics of human skin allow bacteria to colonise it?
|
>Hair increases humidity and temperature of the skin's surface
>Sebaceous glands secrete sebum used as a nutrient by microbes >Apocrine and eccrine sweat glands affect pH, moisture, nutrients and temperature - Strict anaerobes can grow at these sites |
|
|
|
Describe the environment of the skin and its commensals.
|
>Dry environment with a slightly acid pH
- Gram +ve cocci are common >More bacteria are found in moist areas e.g. scalp, armpits and groin >Anaerobic bacteria e.g. Proprionibacterium Acnes live in sweat glands and release free fatty acids from sebum which inhibit other organisms. |
|
|
|
Describe the environment of the eyes and its commensals.
|
>Tears contain lysozyme which inhibits bacteria but Gram +ve rods (coryneform) bacteria occur.
|
|
|
|
Describe the environment of the nose and its commensals.
|
>Gram +ve cocci - staph epidermidis and aureus, coryneform bacteria, streptococci.
>Healthy individuals may carry MRSA. |
|
|
|
Describe the environment of the GU tract and its commensals.
|
>Staph epidermidis, streptococci, Gram -ve rods, yeasts (candida), gut flora
>Adult women carry lactobacilli |
|
|
|
Describe the environment of the mouth and its commensals.
|
>Rich and complex microbiota
- streptococci - actinomycetes - v. strict anaerobes - gram -ve rods and cocci - spirochaetes - yeasts |
|
|
|
Describe the environment of the stomach and small bowel and its commensals.
|
>Streptococci, lactobacilli, possibly gram -ve potential pathogens (Helicobacter spp.)
>At distal end - coliforms, strict anaerobes (Bacteroides spp.) |
|
|
|
Describe the environment of the large bowel and its commensals.
|
2 distinct populations:
1. Wall microbiota - non cultivable bacteria 2. Lumen microbiota >Gut contains approx 1000 species and is believed to be vital for the development of the gut and systemic immune systems. >Colonisation after birth with bifidobacteria (occurs in breast-fed infants) protects against diarrhoea. >Gut contains the greatest density of bacteria 10^12 in the body and sheds the highest number. |
|
|
|
What is the role of the commensal microbiota?
|
1. Provides colonisation resistance
- Prevents overgrowth and colonisation by extraneous pathogens - Modulates acquisition of external pathogens 2. Supplies micronutrients to the host - Gut microbiota produces vitamin B12 - Oral microbiota releases hydrogen peroxide used in salivary peroxidase antimicrobial system 3. Essential for initial immune priming of host - Development of immune competence against pathogens - Ensures tolerance to food antigens and commensal microbiota |
|
|
|
How may commensal microbiota cause disease?
|
>If the flora is disrupted:
- it hasn't achieved stability or maturity - antibiotics >Host susceptibility is increased by: - Immunosuppression: HIV, drugs, pregnancy - Other illness - Poor nutrition |
|
|
|
Describe the microbiota of the gut (oesophagus to anus)
|
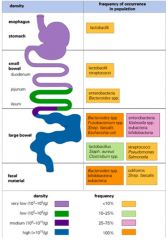
|
|
|
|
Which oral diseases can be caused by commensal microbiota? (5 examples)
|
>Dental caries
>Periodontal diseases >Dento-alveolar abscess >Candidiasis (Thrush) >Sub-acute bacterial endocarditis |
|
|
|
What disease can be caused by overgrowth post antibiotic treatment?
|
>Clostridium Difficile
- Spore forming gram +ve bacillus - Strict anaerobe - lives in gut as minor commensal - Spores survive antibiotics so can overgrow -> serious infection -> death - Pseudo membranous colitis >Picked up from contaminated surfaces |
|
|
|
How are infections acquired by individuals in a population?
|
Infection takes place due to the:
>Host: - A population may have 'herd immunity' e.g. immunity transferred to the whole community - Susceptibility of individuals vary due to genetics, previous exposure, underlying medical condition, exposure to infection, and physical or mental stress >Microorganism: - Most are harmless to healthy individuals - Microbes able to cause damage are termed 'virulent' >Environment: - Overcrowding and poor sanitation encourage rapid spread of infections - Spread of infection is influenced by building design and air conditioning systems e.g. TB hospitals are cliff-facing. |
|
|
|
Give an example of how anatomy might make one more susceptible to an infection.
|
E.g. UTIs are much more common in females due to their GU morphology compared with males.
|
|
|
|
Describe common routes of infection.
|
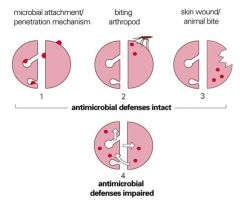
>Microbial attachment / penetration:
- Skin - Respiratory tract (provides the creates opportunity for infections in the body) - GI tract - GU tract >Biting arthropod >Skin wound/animal bite >Commensal microbiota where antimicrobial defences are impaired >Vertical transmission (rare) |
|
|
|
What are common sources of UTIs?
|
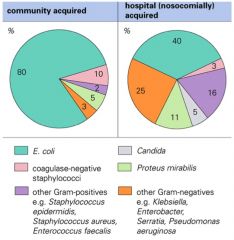
|
|
|
|
Describe how the GI tract may become infected.
|
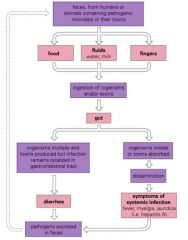
>Infections arise via milk or food from infected animals, or that have been contaminated by unclean water or unwashed hands
>Localised infections usually result in diarrhoea >Systemic infections often cause fever, headache and/or jaundice NB. Infection may be toxin related |
|
|
|
List some examples of STDs, their genus/species and their general characteristics.
|
>Syphilis - Treponema Pallidum
>Gonorrhoea - Neisseria Gonorrhoeae >Chlamydia Trachomatis >Chancroid - Haemophilus ducreyi >Trichomonas vaginalis >Genital Herpes (HSV) >Genital warts >HIV - There is no exposure to the environment with STDs so pathogens do not withstand drying, sunlight and often need extra CO2 to grow. - Syphilis and gonorrhoea can be readily treated with penicillin if they are detected but they may be overlooked. NB. Gono resistance is developing towards penicillins. |
|
|
|
How is malaria transmitted?
|
>Normally by the bite of a female 'anopheline' mosquito which needs a supply of blood in order to produce and lay eggs
>Malaria can also be transmitted by: - Blood transfusion - Contaminated needles and other sharps - Rarely, malaria can be transmitted from mother to child before and or during birth |
|
|
|
Describe different routes of bacterial transmission from person to person.
|
>Aerosol spread:
- smaller droplets travel further - dry aerosol products may float in the air >Hand to nose: - Most common means of rapid spread of infection >Respiratory or salivary spread: - not readily controllable >Faeco-oral spread - controllable by public health measures >Sexually transmitted: - difficult to control as social factors involved |
|
|
|
Which viruses may be transmitted by infectious aerosols?
|
1. Influenza
2. Rhinovirus 3. Adenovirus 4. Mumps 5. Measles 6. Rubella 7. Varicella Zoster 8. Epstein Barr |
|
|
|
Which bacteria may be transmitted by infectious aerosols?
|
>Bordetella pertussis
>Corynebacterium diptheriae >Haemophilus influenzae >Mycobacterium tuberculosis >Mycoplasma pneumoniae >Neisseria meningitidis >Streptococcus pyogenes >Streptococcus pneumoniae |
|
|
|
Describe how hospital cross infection can be spread and controlled.
|
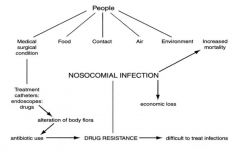
|
Patient evaluation: medical history
Personal protection: immunisation/protective clothing Instrument sterilisation Surface and equipment disinfection Working methods Waste disposal |
|
|
Describe some common routes of bacterial transmission (diagram).
|
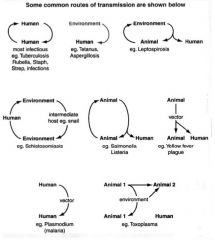
|
|
|
|
Describe the systemic and local spread of gonorrhoea.
|
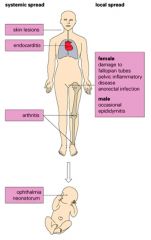
>Not always perceived as a serious infection there can be serious systemic disease in the mother and severe conjunctivitis can occur in her infant after birth.
|
|
|
|
Describe different routes and types of zoonotic infection.
|
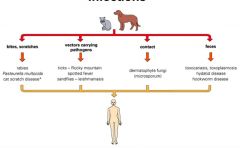
|
|
|
|
Describe the pathway for toxoplasmosis infection.
|
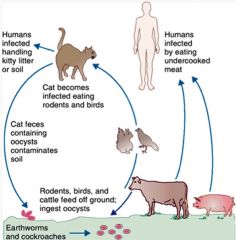
>Acquired by eating cysts in undercooked meat or cat faeces.
>Parasite spreads via the blood. >Usually controlled by the host immune response. >Congenital infections of the foetus can lead to severe complications. |
|
|
|
Define what is an antibiotic and some examples of antibiotic producing microbes.
|
>Antibiotic: Substance produced by a microorganism that in small amounts inhibits the growth of another microbe.
Antibiotic producing microbes: >Gram +ve rods: - Bacillus Subtilis: Bacitracin - Bacillus Polymyxa: Polymyxin >Fungi: - Penicillium notatum: Penicillin - Cephalosporium spp.: Cephalothin >Actinomycetes: - Streptomyces venezuelae: Chloramphenicol - Streptomyces griseus: Streptomycin - Streptomyces nodosus: Amphotericin B - Micromonospora purpurea: Gentamicin |
|
|
|
List 5 examples of antibacterial mechanisms of action.
|
1. Cell wall synthesis inhibition
2. Protein synthesis inhibition 3. Plasmamembrane toxicity 4. Inhibition of nucleic acid synthesis 5. Inhibition of essential metabolite synthesis |
|
|
|
Describe the effect of cell wall synthesis inhibition.
|
>Inhibits peptidoglycan synthesis:
- Results in cell lysis - Low toxicity E.g. Penicillin (by acting as a peptide analogue, inhibiting the enzymes) and Vancomycin (prevents wall subunits joining together) |
|
|
|
Describe the effect of protein synthesis inhibition.
|
>Interfere with prokaryotic (70S) ribosomes (also found in mitochondria)
- Most have broad spectrum of activity - E.g. Tetracyclin, Chloramphenicol, Erythromycin and Streptomycin |
|
|
|
Describe the effect of plasma membrane toxicity.
|
>Causes changes in membrane permeability:
- Results in loss of metabolites and or cell lysis - Many polypeptide antibiotics - E.g. Polymyxin B (antibacterial) or Miconazole (antifungal; specific to ergosterol) - Toxic antibiotics due to presence of cell membranes in microbes and eukaryotic cells. NB. Polymyxin D (Colistin) is highly reno and CNS toxic, so is only used as a last resort in resistant bacterial infection. |
|
|
|
Describe the effect of nucleic acid synthesis inhibition.
|
>Interferes with DNA replication and transcription:
- Inhibits DNA gyrase and topoisomerase - E.g. Quinolones (Cipro) |
|
|
|
Describe the effect of essential metabolite synthesis inhibition.
|
>Involve competitive inhibition of key enzymes:
- Closely resemble substrate of enzyme - E.g. Sulpha drugs inhibit the synthesis of folic acid (here never given to pregnant women because neonates can't develop folate) NB. Folate is a precursor to purines and pyrimidines. |
|
|
|
What influences antibiotic efficacy?
|
>Distribution (pharmacodynamics): in the body; membrane permeability - lipid solubility/active transport
- Concentrations in tissues, bone, CSF, bile etc. >Metabolism (pharmacokinetics): can the antibiotic be broken down by the patient's body and rendered ineffective? >Excretion (liver, kidneys): is the drug removed by the patient's own purification systems before it can be effective. NB. Failure of systems can produce toxicity; dosage must ensure sufficient therapeutic concentration; some drugs e.g. amino glycosides require monitoring such as the aminoglycosides. |
|
|
|
How are antibiotics typically administered?
|
>External (topical) or systemic (blood stream)
>Systemic drugs can be: - Intravenous - IM - Oral >Frequency of doses can vary |
|
|
|
What are the primary safety concerns over using antimicrobials?
|
>Toxicity (kidney/liver damage)
>Interactions with other medications (Rifampicin neutralises effectiveness of contraception - via priming of CYP450) >Hypersensitivity reactions (anaphylaxis to penicillin - doesn't preclude all β-lactams) >Foetal damage/risk to pregnant women (tetracycline causes discolouration of teeth in children and may cause liver damage in pregnant women; fluoroquinolones may cause cartilage damage) >Antibiotic resistance |
|
|
|
How are enzymes becoming resistant at a cellular level?
|
>New enzyme that degrades antibiotic:
- β Lactamases, aminoglycoside modifying enzymes >New pump that pumps out antibiotic - Mef (macrolides); Tet A-E (tetracyclines); MexABOprm (Carbapenems) >Loss of channel used by antibiotic for entry: - D2 porin (carbapenems) >New protein with lower affinity for antibiotic - PBP2a (flucloxacillin), Rpob (rifampicin) >New enzyme/pathway allowing bypass - Folate biosynthesis (trimethoprim) |
|
|
|
How has antibiotic resistance occurred?
|
>Through random genetic events:
- Acquisition of DNA: Plasmids, Transposons and Naked DNA - Alteration of DNA: mutation - Loss of DNA: deletion NB. Staphylococcus aureus strains are all resistant to antibiotics now. |
|
|
|
How can doctors control antibiotic resistance?
|
>Resistance usually carries a biological cost to the micro-organism
- decreased fitness - biological disadvantage in the absence of antibiotic pressure (additional protein, loss of transport pathway, additional energy expenditure, less efficient enzymes/pathways >Overwhelming selective advantage under antibiotic pressure. >By carefully exerting antibiotic pressure doctors can control the selection of bacteria. |
|
|
|
What are the main groups of antibiotics?
|
>β lactams
>Macrolides >Glycopeptides >Aminoglycosides >Quinolones >Anti-folates |
|
|
|
Which antibiotics are considered 'lone rangers'?
|
- Rifampicin (contraind. for contraceptive pill users)
- Sodium fusidate - Chloramphenicol - Doxycycline - Nitrofurantoin - Metronizadole |
|
|
|
Which new and rarely used antibiotics are there?
|
>Linezolid
- Glycopeptide resistant enterococci - VISA / VRSA >Daptomycin - VISA / VRSA >Tigecycline - ESBLs and MRSA |
|
|
|
What are the β-lactams?
|
>Class of antibiotics with three groups:
1. Penicillins 2. Cephalosporins 3. Carbapenems >Inhibit enzymes involved in cell wall assembly >Have no activity agains atypical organisms: - Chlamydia - Mycoplasma - Legionella |
|
|
|
What are the β lactamases?
|
>Enzymes which confer resistance to bacteria against β lactam antibiotics:
- Differ in substrate specificity and inhibitor susceptibility - New variants constantly emerge - Often plasmid mediated (easily transfer between bacteria) |
|
|
|
Which bacteria produce β lactamases?
|
>Some bacteria never produce β-lactamase
- gram +ve rods e.g. listeria/corynebacteria, streptococci, neisseria meningitidis, treponema, most anaerobes >Main producers: - Staph aureus and coagulase negative staph. - Gram negative rods including Haemophilus influenzae - Bacteroides fragilis (gram negative gut anaerobe involved in abdomen-pelivic sepsis) >Some gram negative cocci (gonorrhoea) |
|
|
|
Which penicillins are becoming less effective?
|
>Beta lactamase labile:
- Penicillin - Ampicillin - Amoxicillin - Ticarcillin - Piperacillin |
|
|
|
How does beta lactam resistance manifest?
|
>Alteration in target site affinity for transpeptidase and d-ala decarboxyl transpeptidase
>Beta lactamase production (breaking open of the lactam ring). |
|
|
|
What would give a positive coagulase test?
|
>Staphylococcus aureus
- Highly virulent - Wide variety of infections |
|
|
|
How can the issue of β lactamases be overcome?
|
>Through use of inhibitor combinations such as:
- Co amoxiclav (abdomino pelvic sepsis, pyelonephritis, pneumonia) - Piperacillin Tazobactam - Ticarcillin Clavulanate - These can have issues with penetration and inhibitor susceptibility. >Through stabilising β lactams - Flucloxacillin (modified penicillin stable against staphylococcal β lactamase; staph aureus infections) - Cephalosporins - Carbapenems |
|
|
|
How can loss of target site affinity be overcome?
|
>Different β lactams attack different peptide binding proteins
- possible to use an alternate β lactam e.g. cephalosporin >Some bacteria lose affinity to all β lactams - use non β lactam antibiotic (e.g. glycopeptides, aminoglycosides, doxy, rifampicin, trimethoprim, sodium fusidate) |
|
|
|
List some examples of the superbugs (antibiotically resistant strains).
|
>Multi-drug resistance Mycobacterium tuberculosis
>Methicillin resistant Staphylococcus aureus >Extended-spectrum β lactamase producing enterobacteriaeccae >Penicillin resistant Streptococcus pneumoniae >Penicillin resistant Neisseria gonorrhoeae >Vancomycin resistant Enterococcus faecalis >Multi resistant Pseudomonas and Acinetobacter >Ciprofloxacin resistant Salmonella typhi >>Vancomycin Resistant MRSA |
|
|
|
What are the main causes of antibiotic resistance?
|
>Over the counter antibiotics
>Misuse of antibiotics by doctors >Antibiotics in animal feed |
|
|
|
Give an overview on the cephalosporins.
|
1st generation (cefradine, cefalexin, cefadroxil):
- Gram +ve organisms (incl. β lactamase producers); NOT enterococci. - Some β lactamase negative (e. coli, klebsiella, proteus) 2nd generation (cefuroxime, cefaclor): >Good gram +ve activity - NOT enterococci >Enterobacteriaceae >Some β lactamase stability - cefuroxime stable to TEM-1 3rd generation (cefotaxime, ceftriaxone, ceftazidime): >High risk of C. diff. infection >Activity - Moderate to poor anti-staphylococcal activity - Good activity against most other gram +ve organisms (NOT enterococci) - Very good activity against ESBL negative Enterobacteriacea >Ceftazidime has anti pseudomonal activity. |
|
|
|
Give an overview on the carbapenems.
|
E.g. meropenem, imipenem, ertapenem:
>Very broad spectrum >β lactamase stable >Are vulnerable to resistance due to loss of penicillin binding protein affinity. - Gram positives (not MRSA) - Anaerobes - Gram negatives |
|
|
|
Give an overview on the amino-glycosides.
|
>Aminoglycosides include:
- Gentamicin - Amikacin - Streptomycin - Tobramycin >Activity: Inhibit ribosomal protein synthesis - Staph. incl. MRSA - GRAM NEG BACILLI (enterobacteriaeceae, pseudomonas aeruginosa) - Mycobacteria - Aerobes >Poor tissue penetration >Resistance growing amongst gram +ves >Oto and nephrotoxic; requires close monitoring |
|
|
|
Give an overview on the quinolones.
|
Quinolones (ciprofloxacin, moxifloxacin, levofloxacin):
MoA: >Inhibit DNA gyrase and topoisomerase >Activity: - Gram +ves (not MRSA/enterococci) - GRAM NEG. enterobacteriaceae, pseudomonas aeruginosa, neisseria - Atypicals: Mycoplasma, legionella, chlamydia - Mycobacteria - Aerobes >Excellent tissue penetration (not CSF) >Growing resistance, 20% of enterobacteriaceae are resistant. |
|
|
|
Give an overview on the macrolides.
|
Erythromycin:
>Largely redundant Clarithromycin: >Better absorption, tolerance, PK/PD MoA: Inhibit amino acid binding during protein synthesis Activity: >Gram +ves (β lactam allergy) >Some anaerobes >Atypicals >Mycobacteria (clarithromycin) |
|
|
|
What are azithromycin and clindamycin?
|
>Macrolide related agents: stronger and good penetration in comparison.
|
|
|
|
Give an overview of Glycopeptides.
|
E.g. Vancomycin and Teicoplanin
MoA: >bind to the D-ALA-D-ALA terminal end of peptidoglycan precursors. - this inhibits the action of transglycosidase and transpeptidases (required for cross-linking of peptidoglycans - basic building block of cell wall) - inhibits RNA synthesis Resistance: >Due to substitution of D-Ala with D-lactate which prevents glycopeptide binding Activity: >Gram +ves >C. diff. PK/PD: >Poor tissue penetration Tolerability: >Nephrotoxic |
|
|
|
Give an overview of antifolates.
|
E.g. Sulphonamides, Trimethoprim, Co trimoxazole, Dapsone
Activity: >Sulphonamides - broad spectrum >Trimethoprim - Enterobacteriaceae (15% resistance) - MRSA adjunctive therapy >Co trimoxazole: - Pneumocystis jirovecii >Dapsone: Leprosy MoA: inhibit nucleic acid precursor synthesis Tolerability: >Sulphonamides: - Contraindicated in G6PD deficiency - Allergic reactions >Co trimoxazole: - Myelotoxic (restricted use) |
|
|
|
How common is β lactam allergy?
|
Many patients claiming penicillin allergy are not:
>7-23% true allergic Risk of anaphylaxis with cephalosporins: >0.1-0.0001% Risk of cross reactivity overrated: - 0.1% with 1st generation cephalosporins |
|
|
|
Which antibiotics are bactericidal?
|
>β lactams
>Aminoglycosides >Fluoroquinolones >Rifamycins |
|
|
|
When did antibiotics come about?
|
1940s - introduction of penicillin and sulphonamides.
|
|
|
|
Which antibiotics are bacteriostatic?
|
>Tetracyclines
>Trimethoprim |
|
|
|
What is meant by concentration dependent killing?
|
>Some antibiotics exert bactericidal action that is related to the peak therapeutic concentration reached.
>Aminoglycosides and fluoroquinolones |
|
|
|
What is meant by time dependent killing?
|
>Bactericidal action is related to the length of time an antibiotic exceeds the MIC, and not necessarily related to the peak concentration reached.
|
|
|
|
What is meant by PAE?
|
>Post antibiotic effect:
- bacterial regrowth is inhibited for several hours after the antibiotic concentration has fallen below the MIC. |
|
|
|
Describe the routes of administration of antibiotics.
|
>Per Orum
>Per Rectum >Topical >Intra Muscular >Intra Venous >Nebulised |
|
|
|
What are the key pharmacokinetic factors to consider when choosing antibiotics?
|
>Absorption:
- tetracyclines can be chelated by calcium containing preparations, preventing absorption >Half life: - variable (hours to days) >Protein binding within the bloodstream and ECF >Volume of distribution and tissue concentrations >Penetration into difficult sites (CSF, bone) >Metabolism (metabolite activity also) >Excretion (renal, biliary) >Other drug interactions |
|
|
|
How can antibiotic use be reduced?
|
>Avoiding prescription for likely viral infections
>Gather knowledge of specific microbe(s) before treatment if patient not too ill >Use narrow spectrum antibiotics first >Treat as short as possible e.g. uncomplicated cystitis = 3 days >Review prescriptions at 48h taking note of condition and microbiology results >Rationalise broad spectrum antibiotics on the basis of microbiology results >Avoid unnecessary prophylaxis >Regularly updated antibiotic guidelines should be available >Patient history should be taken into account |
|
|
|
When is antibiotic prophylaxis indicated? Use examples.
|
>Significant risk of infection
>Consequence of infection may be serious >Period of highest risk can be ascertained >Microbial causes can be predicted >Antimicrobial sensitivity of infection is predictable >Cheap and reasonable safe antimicrobial agents are available Examples: 1. Co amoxiclav (prior to surgery e.g. colorectal) 2. Amoxicillin (prior to dental surgery) 3. Rifampicin (contact with meningococcal disease) |
|
|
|
What is the difference between intrinsic and acquired antibiotic resistance?
|
>Intrinsic resistance depends on the natural properties of the bacterium e.g. cell wall impermeability to an antibiotic
>Acquired resistance occurs when the bacteria evolves to resist antibiotic pressure e.g. via mutation, transfer of resistance genes e.g. β lactamase production. |
|
|
|
What are side effects of different antibiotics?
|
>Common reactions include skin rashes, diarrhoea, nausea, vomiting due to upset of natural microbiota
>Specific examples: - Aminoglycosides, nephro- ototoxicity - Co amoxiclav, cholestatic jaundice - Fluoroquinolones, tendonitis, tendon rupture, seizures - Rifampicin, disturbed LFTs (inhibits contraceptive pill effect) - Chloramphenicol, aplastic anaemia - Sulphonamides, Steven-Johnson syndrome |
|
|
|
What factors influence establishment of infection?
|
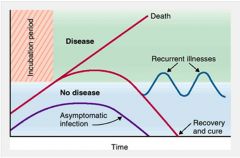
>Minimum infective dose
>In vivo growth rate of pathogen >Pathogen virulence factors >Route of entry >Integrity of host skin and mucosal surfaces >Host: overall state of health and effectiveness of innate and adaptive immune mechanisms |
|
|
|
How may pathogens attach to host cells and what are the consequences of bacterial adherence?
|
>Enteropathogenic E. coli (EPEC) produce pili and adhesins
- Aids adhesion to brush border of intestinal mucosa - Causes local destruction of microvilli >Enterotoxic E. coli (ETEC) possess pili which enable the organism to adhere to mucosal epithelial cells via specific receptors - ETEC produce plasmid-associated enterotoxins POTENTIAL CONSEQUENCES: >No effect >Altered morphology - Invagination of cells to hide from immune defences e.g. pits - Enabled via interaction by Actin cytoskeleton >Cytokine release >Apoptosis >Invasion |
|
|
|
What are typical mechanisms of host damage during bacterial infection?
|
>Lysis of host cells:
- Bacterial toxin that damages cell membrane - Intracellular fission leading to lysis - Programmed cell death (apoptosis) induced by bacteria >Toxin-induced metabolic changes >Mechanical causes - sufficient numbers may cause obstruction >Damage caused by host responses (immunopathology) - e.g. sepsis: cells produce superoxides which will damage vascular endothelium, resulting in puerpera (blood leaking out) |
|
|
|
What are the virulence factors of Strep Pyogenes?
|
>M-protein (adhesin)
>Hyaluronic acid capsule - antiphagocytic >Lipoteichoic acid - antiphagocytic >Erythrogenic toxins A, B, C - superantigens >Hyaluronidase - spreading factor >Streptolysin - O, S - Haemolysins >DNases, hyaluronidase, proteases - virulence enhancers >Pyrogenic exotoxins - rash of Scarlet fever >Toxic shoc syndrome (TSS) |
|
|
|
Describe the mechanism of inhibiting complement.
|
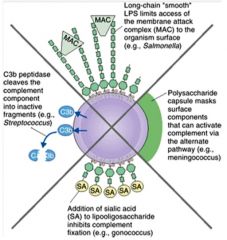
>Pathogens may mask surface components that activate the alternative pathway
>'Smooth' strains of G-ve bacteria hinder access of the MAC to its target >Bacterial enzymes can digest complement components |
|
|
|
Describe the mechanisms of enzymatic lysis and pore formation by bacteria.
|
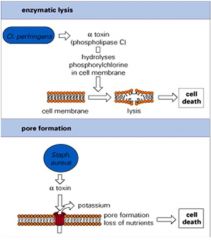
ENZYMATIC LYSIS:
>Clostridium perfringens is a G+ve anaerobic rod - Can cause gangrene - Produces alpha toxin which hydrolyses phosphorylchlorine in the cell membrane - Leads to lysis of the cell; allowing nutrients out for the bacteria PORE FORMATION: >Staph and strep haemolysins are pore forming toxins and can lyse many other types of cell - Knock holes in red cells to absorb iron |
|
|
|
Give examples of pathogens causing local and invasive infections and their presentations.
|
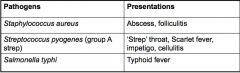
|
|
|
|
Give examples of pathogens causing local and invasive infections and their presentations.
|
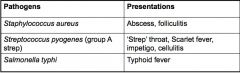
|
|
|
|
Where is staph aureus typically found and what are its virulence factors?
|
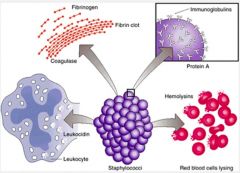
>Commensal of skin and nasal passages
- usually causes local purulent abcesses >Factors include: - Capsule and slime - antiphagocytic - Protein A - binds Fc terminues of IgG - Lipoteichoic acid - adhesin - Pore forming toxins - damage host cells - Panton Valentine leucocidin (PVL) kills neutrophils - DNAses, lipases and proteases enhance virulence - Beta lactamase for antibiotic resistance - Exfoliative toxins A and B - scalded skin syndrome - Enterotoxins - food poisoning - TSS - toxic shock toxins |
|
|
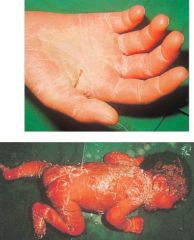
What might this be an effect of?
|
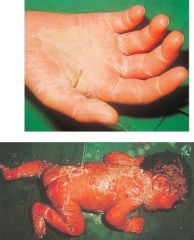
>Staphylococcal Exotoxins:
- Desquamation of hand due to TSS - Scalded syndrome |
|
|
|
Describe the symptoms of S. Pyogenes infection and its virulence factors.
|
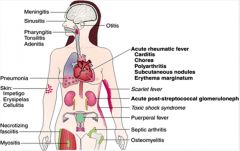
>Virulence factors:
• M-protein – adhesin, anticomplementary • Hyaluronic acid capsule – antiphagocytic • Lipoteichoic acid – adhesin • Erythrogenic toxins A,B,C – superantigens • Hyaluronidase – spreading factor • Streptolysin, O, S – haemolysins • DNases, hyaluronidase, proteases – virulence enhancers • Pyrogenic exotoxins – rash of Scarlet fever • Toxic shock syndrome (TSS) toxins – TSS |
|
|
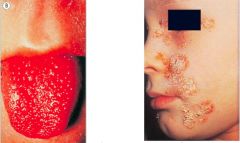
What is this pathology and what might it be caused by?
|
>Clinical streptococcal disease
- Scarlet fever with rash and strawberry tongue - Impetigo - often with staph aureus |
|
|
|
Describe the course of S. Typhi infection and its virulence factors.
|
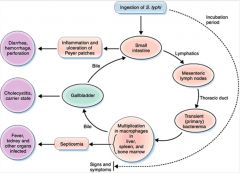
• G-ve facultative anaerobic rods
• The main antigens are somatic (O), flagellar (H) and capsular (K) • There are many other species which infect animals and can cause food poisoning in humans • Specific human pathogen • Penetrates the mucosal barrier in the distal ileum and colon by bacterial-mediated endocytosis • Resist lysosomal digestion and pass to the lymphatic system. • Can survive and grow within phagocytes |
|
|
|
Which 3 ways are bacteria typically pathogenic?
|
>Via exotoxins
>Invasion >Immunopathology |
|
|
|
What are exotoxins?
|
>Toxins which give rise to a recognisable illness
- have specific cellular target; - or act upon host cell membrane leading to cell death and necrosis - may be involved in intracellular invasion of host cells by pathogen - in excess cause activation of the immune system |
|
|
|
Which toxins act on cell surfaces?
|
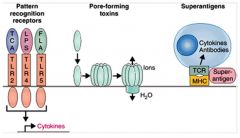
>PAMPs
- e.g. Teichoic acid, LPS and flagellin >Pore forming toxins >Superantigens |
|
|
|
Describe Diptheria toxin's mode of action?
|
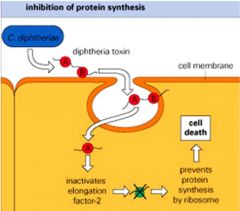
>C. diptheriae is a G +ve aerobic club shaped rod
>Toxin genes are carried by a lysogenic bacteriophage integrated into the bacterial DNA >Toxin producing strains of Corynebacterium diphtheria can produce 5000 toxin molecules/hour >One molecule can kill a host cell |
|
|
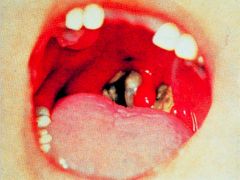
What is this disease and its progression?
|
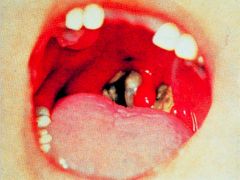
>Bacteria multiply locally
>Toxin kills cells and an an ulcer forms with a necrotic exudate covered by a false membrane >Death can occur due to respiratory obstruction or heart failure >Immunisation with toxoid (inactive) prevents disease >Treatment with antitoxin and antibiotics >Surgery potentially necessary to clear the airway |
|
|
|
What is Cholera's mode of action?
|
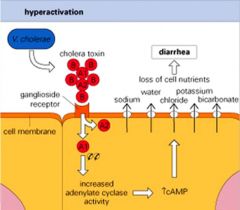
>V cholerae is a G -ve aerobic, comma shaped bacterium
>Vibrio cholerae toxin causes diarrhoea by activation of adenyl cyclase leading to high levels of cAMP in small intestine epithelial cells >This leads to massive transport of fluid and nutrients into gut lumen |
|
|
|
Describe the symptoms of cholera and treatment.
|
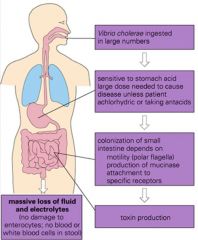
>Rice water stools
>Treatment = Oral rehydration therapy |
|
|
|
Explain the MoA of Tetanus neurotoxin.
|
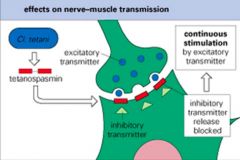
>Clostridium tetani is a G +ve anaerobic spore forming rod
>Tetanus toxin B subunit binds to ganglioside receptors on nerve cells >A subunit then migrates to the CNS and blocks neurotransmitter release leading to continuous stimulation and spastic paralysis (i.e. tetany) What is the course of infection and treatment? |
>C. Tetani found in soil and manure
>Tetanus results from the penetration of a foreign object (garden fork, rusty nail) into the host causing tissue necrosis which allows spores to germinate and produce toxin >Neonates become infected via the cut umbilical cord >Death results from respiratory failure due to paralysis of chest muscles >Immunisation with toxoid prevents disease >Treatment is ant-toxin (must be rapidly given) and antibiotics >Surgical debridement eliminates the focus of infection |
|
|
Describe the action of C. botulinum neurotoxin.
|
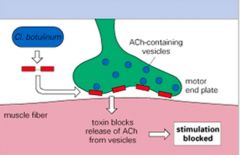
>Enters via the intestine and crosses the gut wall
>Affects peripheral nerve endings at the neuromuscular junction >Blocks peripheral nerve stimulation of muscle contraction leading to flaccid paralysis >One of the most potent toxins known - 400g could kill the entire planet >What is the course of infection with C. Botulinum? |
>Bacterium found in soil and can contaminate foods
- spores survive incomplete heat treatment >Botulism is an intoxication by preformed toxin and food-borne disease is the tropical presentation >Symptoms descend from cranium so lack of response to light in the eye is an early sign (within 6h) >Neonates become ill due to toxin produced in the gut causing floppy baby syndrome >Death results from respiratory failure due to muscle paralysis >Treatment is anti toxin - ventilatory support is vital >Used in the cosmetics industry and in the treatment of migraine |
|
|
How may bacteria induce immune pathology?
|
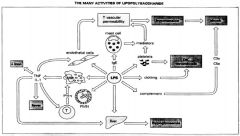
>Bacterial cell components e.g. LPS (endotoxin) of G -ve bacteria activate the host defence mechanisms in a way that causes tissue injury
- LPS binds to LPS binding protein found in plasma - Complex binds to CD14 on macrophage cell membrane which activates it - TNF and IL-1 are released - LPS also activates coagulation complement and kallikrein cascades |
|

