![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
116 Cards in this Set
- Front
- Back
|
General Anesthetics
|
Reversible depression of CNS:
analgesia, loss of consciousness, muscular relaxation, reduced reflex activity. IV; Inhalants, gases and volatile liquids, all highly lipophilic. Structurally nonspecific drugs. Not dependent on activity with any specific receptor, more dependent on physical properties, like partition coefficient (direct correlation). Present Opinion: Protein Hypothesis |
|
|
Protein Hypothesis
|
Agents act "at or near" receptors for inhibitory CNS effects, eg: GABAa. Explains "cut off" phenomenon in which anesthetic potency in a homologous series increases up to a certain point then stops, regardless of increasing logP (lipophilicity). Eventually the size of the molec will exceed the size of the receptor pocket.
Stage1: Analgesia. Stage2: Excitement, amnesia, irregular respiration, vomiting. Stage3: Surgical anesthesia, regular respiration. Stage4: medullary depression, respiration stops. |
|
|
N2O
|
Nitrous Oxide. Gaseous General Anesthetics.
Good analgesia, weak general anesthetic (needs ~80% N2O in O2, too high concentration). Doesn't cause skeletal muscle relaxation, not desired for surgical procedure (alone, at least). Advantage: Not flammable. Needs to be stored in cylinders however; inconvenient. |
|
|
Triangle
|
Triangles. Gaseous General Anesthetics.
Analgesia, general anesthetics Can produce skeletal muscle relaxation Rapid onset ~15min. Organic compound, easily flammable, explosive. |
|
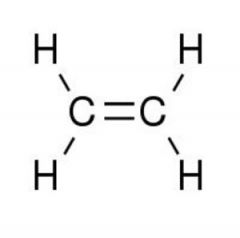
|
Ethylene. Gaseous General Anesthetics
Rapid anesthesia, poor muscle relaxation. Adv: less toxic than cyclopropane, minimal post operative vomiting Organic, easily flammable, explosive. |
|
|
Volatile Liquid General Anesthetic
|
More halogens-->higher BP-->Liquids at RoomTemp
Bottels, better, smaller. Low flammability, enhanced lipid partitioning. Ethers produce desired muscle relaxation. Some of the SEs are related to the introduction of halogens. Cl/Br--> cardiotox; sensitize heart to NE/Epi -->ventricular fibrillation, arrhythmias. F will cause Neph/hepatic tox due to bioaccumulation. You dont want these to be heavily metabolised, will release halogens, will produce tox. |
|
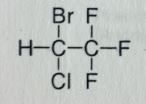
|
Halothane. Volatile Liquid General Anesthetic
Rapid induction/emergence of anesthesia. Moderate muscle relaxation. Racemic; activity doesnt really change though, its more about lipophilicity. BP=50.2 MTBSM: Highest degree within class, ~20%. Formation of TriFlouroAcetic acid (TFA; strong/corrosive). F/Br release--> hepatic necrosis, cardiac arrhythmias. May also undergo elimination, this is accompanied by the release of HF. NOT flammable |
|
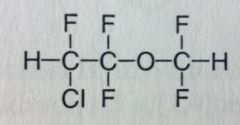
|
Enflurane. Volatile Liquid General Anesthetic
Rapid Ind/Eme from anesthesia. Moderate muscle relaxation. Racemic; ether; BP= 56.5 MTBSM: "C-F" bonds are very strong, less overall mtbsm, ~5% Carboxylic acid with HF release. F's may undergo elimination and potentiate further HF release. |
|
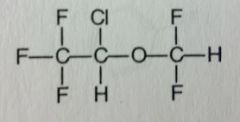
|
Isoflurane. Volatile Liquid General Anesthetic
Rapid Ind/Eme of anesthesia, moderate muscle relaxation. Widely used, very similar to Enflurane (its an isomer of it). Racemic; BP= 48.5 MTBSM: significantly reduced (~0.2%), but still release of HF. <--overall, advantageous. |
|
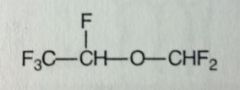
|
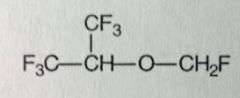
Desflurane. Volatile Liquid General Anesthetic
Rapid Eme/Ind of anesthesia, can be used for out pt use. Ind/Maintenance of anesthesia. Unpleasant odor, irritates airway so not for extended use. Not favorable for induction in kids, can be used to maintain. "even better than isoflurane" "Des"=something removed, Cl, b/c it caused most mtbsm problems. Racemic. BP=23.5, close to RoomT, packaged differently. MTBSM: no more Cl, only strong C-F bonds, mtbsm further reduced, ~0.02%. |
|
|
Sevoflurane. Volatile Liquid General Anesthetic
Ind/mtn/eme of anesthesia. muscle relaxation. More pleasant smell, less irritation, more suitable for Peds. More halogens, heavier, higher BP ~58.6 MTBSM: elimination with HF release. ~5%, problematic. |
|
|
IV Anesthetics
|
No special equipment required, just a syringe.
Faster onset, more rapid recovery. Suitable for outpatient. |
|
|
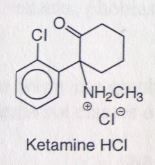
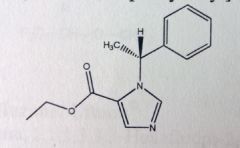
Ketamine HCl. Intravenous Anesthetics.
Dissociative anesthesia (w.o loss of consciousness), rapid induction. No muscle relaxation. Diagnostic tool for procedures where muscle relaxation not needed. Inhibits brain effects of glutamic acid--->inhibits glutamate (antagonist at NMDA receptor). Secondary amine= very basic= high water solubility. Short t1/2 (10-25mins). Chiral center, sold racemic. MTBSM: Oxidative dealkylation (primary amine formed). α-oxidation ( α to ketone, forms a site for conjugation). |
|
|
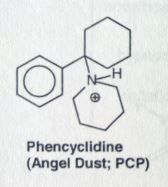
phencyclidine. PCP.
Structurally similar to Ketamine. Explains the hallucinogenic effects of ketamine, that can occur up to 24 hrs after use. |
|
|
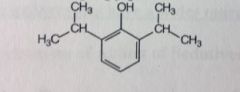
Propofol. Intravenous Anesthetics.
Continuous sedation of adult pts in ICU. Anti-emetic properties. *emesis usually an SE of anesthesia. Is the drug of choice for ambulatory surgery in outpatients. Enhances GABA-ergic neurotransmission (but doesnt bind to bz receptor site). Onset ~40secs, similar to barbiturates. Recovery is faster compared to barbiturates. MTBSM: Phenol-->gluc'd/sulfated (99%) |
|
|
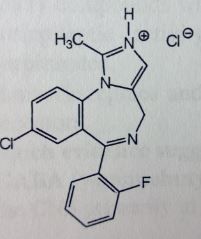
Midazolam. Intravenous Anesthetics.
IV Drug of choice in ambulatory care, surgery in outpts. Amnesia (may be desired effect). Preoperative induction (slower onset and prolonged recovery compared to barbiturates. BZ structure with fused pyrazole (imidazole=basic=salt formed). Binds to allosteric site on GABAa-receptor, a BZ site. MTBSM: Hydroxylation at α-methyl-->gluc'd. Phenyls have e- withdrawing grp--> not susceptible to mtbsm. Flumazenil used in ODs or when effects are too prolonged (b/c its iv). Overcomes effects by antagonism at another allosteric site. |
|
|
Etomidate. Intravenous Anesthetics.
Induces genreral anesthesia, but no analgesic activity. Very quick hypnosis, <1min. Very short acting, used for short procedures NOT requiring muscle relaxation: joint dislocations, cardioversion. Decreased resp depression and cardiac SEs than barbs and thiopentals. MTBSM: ester hydrolysis leads to inactive carboxylic acid containing metabolite. |
|
|
Barbiturates
|
Classified based on their DoA.
Methohexital/Thiopental Sodium are ultra-short acting barbiturates. Are more relatively more lipophilic compared to other barbs. Quickly cross the BBB, fast onset. But can also quickly leave BBB, short acting. Neither have muscle relaxant properties. The more C's @ 5 position, the more lipophilic |
|
|
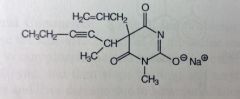
Methohexital Sodium. IV Anesthetics. Ultrashort barb
Rapid onset, ultra short acting, DoA <20mins Methyl @ N increases lipophilicity. No muscle relaxation |
|
|
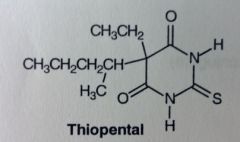
Thiopental (Sodium). IV Anesthetics. Ultrashort barb
Most common barb used to induce anesthesia. No muscle relaxation. Two different carbon chains @ 5', and replacing carbonyl with sulfur increases lipophilicity (less electronegative than O). t1/2 is 3hrs, somewhat prolonged b/c it uses fat as a depot---> prolonged anesthesia. still, its short acting compared to other barbs. MTBSM: After admin'd, sulfur removed as sulfate (if oxidized) or H2S (if reduced). Major active metabolite produced is pentobarbital (marketed on its own). |
|
|
Sedative-Hypnotic Agents
|
1. Sedation= calming effect w/ little or no effect on motor or neural function; decreases response to constant level of stimulus.
2. Hypnotic= produces drowsiness; promotes onset & maintenance of sleep (as close to natural as possible). All sedative agents produce hypnotic effects at high enough doses. 3. Anxiolytic= specifically relieves excess anxiety or apprehension that is excessive or inappropriate to the situation (panic attacks, phobias). |
|
|
Barbs & Benzos, Function of dose.
|
Sedation= small dose, minimal CNS depression. Hypnotic effects require greater CNS depression. Further increases in depression lead to general anesthesia.
*Beyond this point= medullary depression (resp & vasomotor centers)--> coma & death. From graph: BZs have a wider margin of safety; thier effects taper off, while barbs have linear function relationship. *Anxiolytic/anti-seizure effects are found at lowest dose (Iba 2). |
|
|
Sedative-Hypnotic MoA
|
Major classes: barbs & benzos.
Many agents also have antiepileptic activity. Allosteric binding to GABAa-receptor to potentiate GABA's inhibitory effects (*for BZs atleast, unsure of exactly where barbs bind). GABAa-receptor=Ionotropic. Post-snyaptic inhibition via potentiating GABA effects (higher frequency of Cl-channel opening. Leads to IPSPs. Doesn't open the channel itself). GABAb-receptor= Metabotropic. Presynaptic. Autoreceptor. Dec'd Ca++ conductance-->dec'd NT release. (Ca++ needed for vesicular fusion). Stimulation at certain points of spine cause muscle relaxation. |
|
|
GABA pic here
|
gaba mtbsm pic here
*zwitterion, cannot cross BBB, therefore produced in neurons. |
|
|
Barbiturates.
|
Derivatives of barbituric Acid. Imide N's pka~7-8; 5-positon pka~4-5 (<---most acidic position).
5' requires 2subs, if there is only 1, it'll ionize to highly acidic Trihydroxypyrimidine (<---largely ionized, doesn't cross BBB) Methyl at N1--->inc'd lipophilicity, and weaker acid b/c fewer resonance structures for conjugate base. }--> faster onset, dec'd DoA. Subbing sulfur at 2'-ketone gives thiobarbituric acid derivative. Increases lipophilicity. }--> faster onset, dec'd DoA. |
|
|
Barbiturates misc SAR
|
5'-more C's-->inc'd lipophilicity (only up to a max of 7-9 C's). *phenyls count at 3-4 C's.
Faster onset & dec'd DoA due to lipophilicty AND inc's rate of mtbsm. Polar groups, unsaturation, & branching at 5' all increase mtbsm-->dec'd DoA Subs at both N's dec activity. |
|
|
Barbiturate Notes
|
Barbs induce Cyp enzymes, Gluc transferase and, UDPGA system (more gluc's)
Classified by DoA: Ultrashort= <3hrs, general anesthetics. Short= 3-4 hrs, sed-hyps & pre-anesthetics. Intermediate= 6-8 hrs, sedative hypnotics. Long= 10-16hrs, anti-convulstants. *However, generally used as anesthetics/ anti convulsants, since there are better drugs for sed-hyp. |
|
|
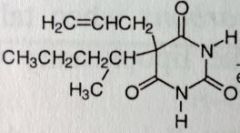
Secobarbital. Sed-Hyps. Short Acting Barbs
Sed-Hypnotic, preanesthetic. Onset: 10-15mins. DoA: 3-4hrs MTBSM: α-oxidation @ allylic position. ω-1 oxidation of sec-pentyl chain. Epoxidation @ dbl bond--->hydrolysis of epoxide to diol. These lead to inactivation of Secobarbital. Epoxidation reaction has potential to inactive cyp enzymes by alkylating them. |
|
|
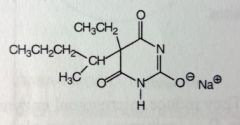
Pentobarbital Soudium. Sed-Hyps. Short Acting Barbs.
Same general use as Secobarbital (b/c same SAR). Sed-Hypnotic, preanesthetic. Onset 10-15mins. DoA: 3-4hrs MTBSM @ points of branching. |
|
|
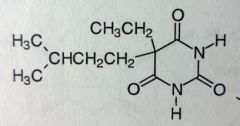
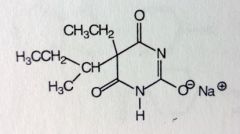
Amobarbital. Sed-Hyps. Intermediate Acting Barbs
Sed-Hypnotic Onset: 45-60mins. DoA: 6-8hrs MTBSM: ω/ω-1 oxidation of isopentyl group-->inactivation. |
|
|
Butabarbital. Sed-Hyps. Intermediate Acting Barbs
Sed-hypnotic Onset: 45-60mins. DoA:6-8hrs Increasing DoA as # of C's decrease. |
|
|
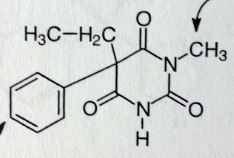
Mephobarbital. Sed-Hyps. Long Acting Barbs
Sed-hyp. Anticonvulsant, Grand mal seizures (b/c long acting) <--main use. Methyl @ N increases lipophilicty slightly, allows for quicker onset than phenobarbital, but DoA is same b/c once its demethylated (main MTBSM process) it IS phenobarbital. Can also para-hydroxylate Sold as racemic mix; S-enantiomer more likely to undergo N-demethlyation. R is 7x more likely to para-hydroxylate. |
|
|
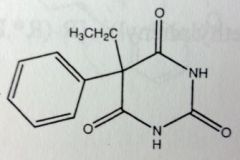
Phenobarbital. Sed-Hyps. Intermediate Acting Barbs
Sed-Hypnotic. Anti-convulsant. Sold Racemic. Para hydroxylation--->glucuronidation. Drug has a plane of symmetry; its a prochiral molecule that will form the R-enantiomer 7:1 |
|
|
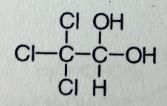
Chloral Hydrate. Misc Sed-Hyp
Sed-hyp, safe for PEDS, but taste bad, give with juice. Prevent or supress alch withdrawal symptoms. Synergistic effects with alch, sedation can be intense, abuse potential, controlled drug CIV Molec is a hydrated acetylaldehyde derivative. The Cl's are electronegative, makes the carbonyl bond very polar and open to attack by water, therefore the hydrate form predominates over the aldehyde form. MTBSM: Hydrate can be oxidized to inactive carboxylic acid (TriChloroAcetic Acid), or reduced to active TriChloroEthanol. Activity belived to be releated to GABAa |
|
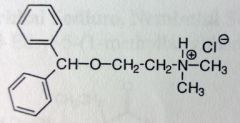
|
Diphenhydramine. Misc Sed-Hyp
General CNS depressant, sleep aid, good for PEDS, OTC. Gen1 antihistamine. Easily crosses BBB, SE: drowziness. |
|
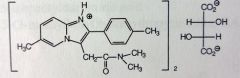
|
Zolpidem Tartrate.
Short term insomnia Tx, 2-6 weeks. Used in armed forces, induces sleep. t1/2=4-6 hrs BZ-1 receptor (sleep), enhances GABAa activity. MTBSM: Less than 10% excreted unhanged. Oxidation of benzyl methyl (80-90%), Imidazo methyl is ~10%--->first ox'd to alch--->aldehyde---->COOH. Can also have some Aromatic hydroxylation @ 7' imidazo carbon. |
|
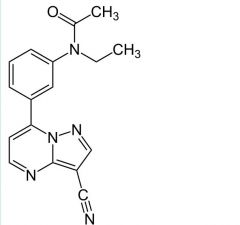
|
Zaleplon
Short term insomnia Tx BZ-1 binding t1/2= 1hr MTBSM: oxidation of pyrimidine-->5'oxo. N-dealkylation (ethyl) <1% left unchanged |
|
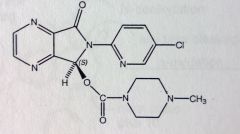
|
Eszopiclone.
Hypnotic Allosteric binding to GABA receptor, not BZ site though t 1/2= 6hrs MTBSM: <10% left unchanged Mostly oxidation at N, the demethylation leaves molec only slightly active. Carbamate is more stable to hydrolysis than the ester or amide alone. |
|
|
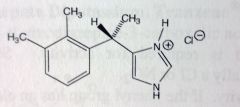
Dexmedetomidine.
Sedative in ICU setting. Adjunct with regional/general anesthetics. Selective α2 receptor agonist-->acts presynaptically, inhibits NE/E release. t1/2= 2hrs (pretty short) "dex"= S-enantiomer MTBSM: Almost complete mtbsm, oxidation at phenyl methylation, OH-->CHO-->COOH. More likely at 3methyl, its more exposed. Imidazole N3-->gluc'd |
|
|
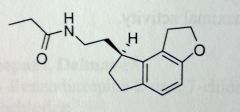
Ramelteon
PO. Insomnia, suitable for longet term use. Melatonin analog, binds to MT1, MT2 receptor, unlike the GABA allosteric receptor drugs. We dont sell melatonin b/c of variable BA. Changes from melatonin: acetamide-->proprionamide. Indole-->hydrocarbon ring system with similarity to methoxy, which is now better held in place. ~85% abs'd--->extensive 1st pass mtbsm--->1.8% BA. MTSBM: Hydroxylation α to amide, retains only 10% activity, but appears with ~100x concentration of parent drug--->Mild sleep inducing activity. |
|
|
Benzos SAR
|
e- withdrawing grp at 7' needed for activity, usually Cl/N2O
Phenyl needed at 5', usually has e- withdrawing group (Cl/F) at ortho or both ortho positions to inc activity. Sub at para would decrease activity. Saturating the 4,5 dbl bond (imine) dec's activity. OH at 3' is tolerated 2' carbonyl is optimal for activity Small sub (methyl/ethyl) is optimal for activity BZ-1,2,3 allosteric sites on GABAreceptor. All found in CNS, 3 is also seen in periphery. 1=sleep, 2= anti-anxiety. |
|
|
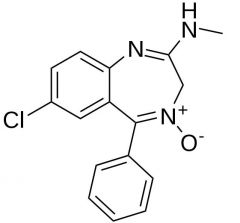
Chlordiazepoxide HCl. Benzodiazepine.
Anxiolytic, sedative one of the first benzos MTBSM: N-dealkylation, then possible hydrolysis of amide to lactam. α-oxidation @3'. reduction of oxide to imine. *hasnt been on an exam in years. |
|
|
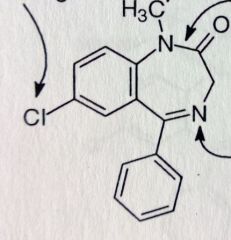
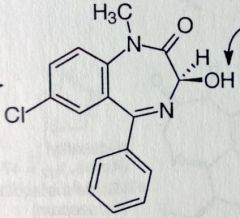
Diazepam. Benzodiazepine.
Axiolytic, antiepileptic, active alch withdrawal symptoms, muscle relaxant (*relieves skeletal muscle spasms, tetanus, spasticity caused by cerebral palsy and paraplegia). up to 100hrs t1/2 MTBSM: para-hydrox--->inactive. Possilbe amidase cleavage. N-dealkylation. α-oxidation leads to 3'OH-->metabolite is marketed as Temazepam. If Temazepam is N-demethylated-->Oxazepam |
|
|
Temazepam. Benzodiazepine.
Hypnotic (OH introduced from Diazepam) Can now conjugate with gluc Can now Demethylate to Oxazepam: Anxiolytic, active alch withdrawal symptoms. |
|
|
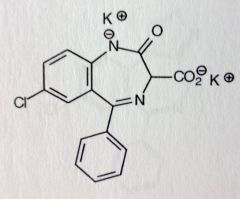
Clorazepate Dipotassium. Benzodiazepine. Prodrug
Inactive until decarboxylase converts it to active Nordiazepam ( =diazepam w/o N1-methyl) Anxiolytic, Antiepileptic, active alch withdrawal symptoms t1/2 = 36-200hrs |
|
|
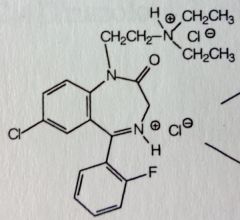
Flurazepam. Benzodiazepine. Prodrug
Insomnia MTBSM: Large N1 sub, needs to be small to be active drug. Deamination produces aldehyde and primary ethyl alch (this is gluc'd and so, contributes little to drug activity and t1/2. major urinary metabolite). Dealkylation leads to active N-desalkyl metabolite with t1/2= 47-100hrs. |
|
|
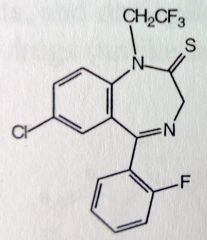
Quazepam. Benzodiazepam. NOT Prodrug
Insomnia. More selective for BZ-1 MTBSM: Reduction of C=S to C=O produces active metabolite. Dealkylation produces same N-desalkyl metabolite as Flurazepam, t1/2 = 47-100 hrs. |
|
|
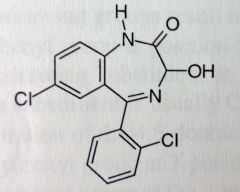
Lorazepam. Benzodiazepam.
Anxiolytic, anti-epileptic. MTBSM: Phenyls are protected by Cl's. They also add lipophilicty, allows for use at lower dose (anxiety, seizures). |
|
|
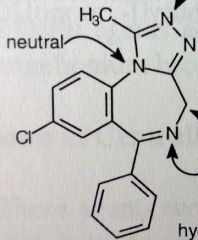
Alprazolam. Triazolobenzodiazepine
Anxiety, panic disorder, agoraphobia Relatively small doses comapered to benzos: 0.25-0.50mg TID MTBSM: α-oxidation @ 4' (same reaction as the 3' α-oxidation, just numbered differently now). Para- hydroxylation of unsub'd phenyl-->conj *[4,3-a] fusion. All N's are basic except middle N, its e-'s are conjugated with aromatic triazole system. |
|
|
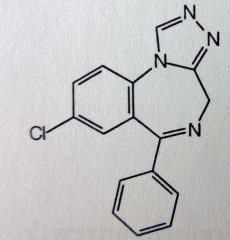
Estazolam. Triazolobenzodiazepine.
Insomnia, 1-2mg qHS. (larger dose, and once a day makes it incompatible for anx/panic therapy). MTBSM: α-oxidation @ 4' Para- hydroxylation of unsub'd phenyl-->conj |
|
|
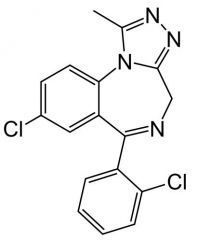
Triazolam. Triazolobenzodiazepine
Insomnia, 0.25mg qHS (smaller, b/c Cl, better lipo) Smaller dose used b/c it has greater activity, and b/c SEs are dose related. Regiment must be individualized per patient (one of narrowest margins of safety), pt starts on LOW dose. MTBSM: additional Cl at ortho will improve activity and prevent para-hydrox (improves t1/2) α-oxidation @ 4' |
|
|
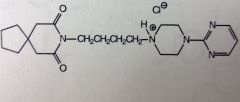
Buspirone HCl.
Short term anx tx. NO antiepileptic/muscle relaxation properties. NO sedatives effects. MoA not well know, known to act in CNS *decane spiro system. The most basic N is the tertiary amine. The one next to the two carbonyls is NOT basic. the other looks like a tertiary amine, but its conjugated |
|
|
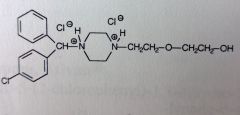
Hydroxyzine HCl.
Gen1 AntiHistamine, Mild tranquilizer. SE: teratogenic activity, birth defects. *Both Piperazine N's are basic--->salt formed. Neither is effected by conjugation. MTBSM: Para-hydrox of the unsub'd phenyl. Primary alch can be ox'd to COOH Ether--->dealkylation possible |
|
|
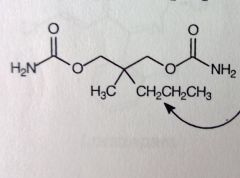
Meprobamate.
Anxiety, "happy pill" MoA: unknown, general CNS supression. Controlled (CIV, b/c potential for abuse) *symmetric molec, therefore NO chiral center. Carbamate N's are NOT basic. Easy BBB penetraion. |
|
|
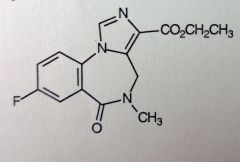
Flumazenil. BZ receptor antagonist
Detox for BZ OD, reverses effect or, used to overcome anesthesia. B/c of ester, initial t1/2 is 7-15mins (20-30mins in brain, after repeated administraion, this is new t1/2). but given slowly IV (200mcg per 1-2mins), repeatedly given as required. MAX: 3mgs/hr. Replaces BZs from BZ-site, by binding instead (competative antagonism), but does not enhance GABA binding once bound. *retains general BZ structure, but w/ imidazole. Carbonyl where phenyl should be, Lactam. F instead of Cl/N2O. @3' carboxylic acid ester--->point of hydrolyis--->inactivation. |
|
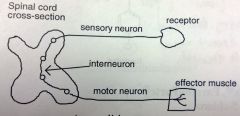
Skeletal Muscle Relaxants
|
A stimulated sensory neuron sends an impulse through the sensory neuron, across the interneuron, and down the motor neuron to effector muscle for contraction.
These agents work by blocking impulses at the interneuron (withing the spinal cord, CNS). Overstimulation causes a spastic condition, so, by blocking at the interneuron you treat: strains; sprains; trauma of the neck, back and joints; spasticity. Since its depressing a component of the CNS, these agents tend to produce sedation, especially in large doses. *these agents are required for some surgical procedures *carbamates have no basicity, more stable to mtbsm |
|
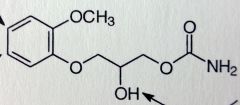
|
Methocarbomal. Centrally-acting Muscle Rxnts.
Relieves disconfort assoc'd with acute and painful muscle contraction; used to control the neuromuscular component of tetanus (from bacterial toxin) Often used with analgesic agents, eg: acetaminophen (Robaxacet); ibuprophen (robax platinum?); Aspirin (robaxisal) MoA; unknown, but related to general CNS suppression *Note: its the carbamate of Guaifenesin, but does NOT convert to guaifenesin. |
|
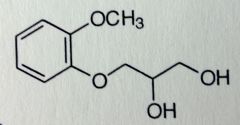
|
Guaifenesin
One of the components of cough medicines, usually a mixture with DXM; expectorant, promotes better clearance of mucus. |
|
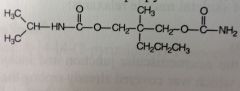
|
Carisoprodol. Centrally-acting Muscle Rxnts.
Muscle spasms, pain. Activity related to CNS depression, exact mech unknown. MTBSM: N-dealkylation--->removal of isopropyl group--> meprobamate formed (anxiolytic, CNS depressive activity) + ketone. Controlled in some states, like meprobamate. *no longer a symmetric molec; has chiral center, sold racemic |
|
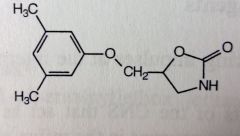
|
Metaxalone. Centrally-acting Muscle Rxnts.
MoA unknown, general CNS depression. * internal carbamate within ring system. |
|
|
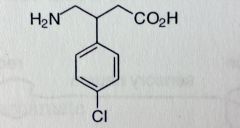
Baclofen. Centrally-acting Muscle Rxnts.
Spasticity as a result of Multiple Sclerosis & spinal cord injury. Less sedation than diazepams. Does NOT reduce muscle strength (unlike Dantrolene). Analog of GABA, Chlorophenyl sub at beta position. Quick absorbtion Agonist for GABAb-receptors (presynaptic, inhibits NT release) MTBSM: very little mtbsm, para Cl prevents hydroxylation, most of the druf is eliminated unchanged. |
|
|
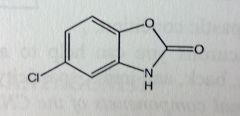
Chlorzoxazone. Centrally-acting Muscle Rxnts.
Sprains, strains, back pains. *Carbamate ring system, fused to benz. Cl reduces metabolic liabilty. |
|
|
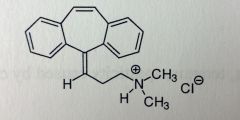
Cyclobenzaprine HCl. Centrally-acting Muscle Rxnts.
Muscle spasms. Tabs(flexeril, fexmid) for TID use. Amrix is QD extended release. 30-50% oral BA, relatively good MTBSM: Extensively metabolized. DBL bond-->Epoxide-->hydrolyzed to diol (dihydroxy-amitriptyline). Tertiary amine can have demethylation, deamination, followed by conjugation. *resembles TCAs, w/o the dbl bond (top, 10,11?), its amitriptyline. W/o 1 of the N-methyls, its Nortriptyline. |
|
|
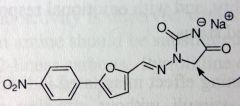
Dantrolene. Centrally-acting Muscle Rxnts.
Control of muscle spasms from stroke, spine injury, multiple sclerosis. Acts peripherally to dec spasticity. No antiepileptic activity *Only Tx for malignant hyperthermia (triggered from volatile anesthetic use, in certain susceptible pts. From drastic increase in oxidative mtbsm in muscle-->not enough O2, too much CO2, rise in body temp, life threatening if not treated). MTBSM: α-oxidation Nitro can be reduced to amine, can also have activated hydroxylamine, which can alkylate DNAse. Usually want to avoid nitroaromatic grps for carcinogenicity. *DOES cause muscle weakening, unlike baclofen. |
|
|
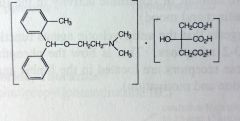
Orphenadrine Citrate. Centrally-acting Muscle Rxnts.
Pain, muscle spasm, leg cramps. Mech: not very well understood for muscle relaxation. *structurally a Gen1 antihistamine, w/o the methyl grp its diphenhydramine. SE: drowsiness b/c of anticholinergic effects. |
|
|
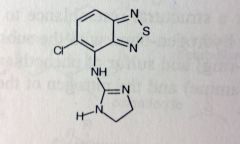
Tizanidine. Centrally-acting Muscle Rxnts.
Decreases excitatory input to motor neurons. skeletal muscle relaxant. Sedative? *structuarly resembles clonidine (HTN), acts as a centrally acting α2 agonist (2-10% blood pressure activity of clonidine). t1/2= 2.5hrs MTBSM: α-oxidation at imidazolidine? 95% mtb'd in liver to the inactive metabolites *note guanidine |
|
|
Antipstchotics (Neuroleptics)
|
Treats agitated states and psychoses like Schizophrenia (disintegration of the thinking process, or contact with reality, with emotional responsiveness).
Reduces euphoria and hyperactivity; produces claming effect and lessens reactivity to emotional stimuli *w/o affecting consciousness* <-- this implies a mech other than CNS depression. Many of the treated conditions are from imbalances in the synth, mtbsm, and distribution of amino NTs (dopamine, NE, seratonin). Schizophrenia is from excessive amounts of dopamine in the Limbic system. Phenothiazines & butyrophenones block dopamine at postsynaptic D2 receptors. |
|
|
Phenothiazines
|
When the sidechain amine is protonated, the phenothiazines bear structural resemblance to dopamine.
The proton H-bonds with the e- withdrawing group at 2'. In this conformation the aromatic ring and the sulfur of the PTAs correlate with the dopamine structure (S w/ the paraOH of dopamine). D2 receptor also has antiemetic properties, many agents used for motion sickness. Agents often present anticholinergic SEs: confusion, constipation, agitation. |
|
|
Phenothiazine SAR
|
Side chain N, most basic site, pKb~5. Diarylamine pKb~ 12-13 (very weak b/c of conjugaiton with ring system.
Neuroleptic potency increases as electronegativity of 2'-sub increases. Max neuroleptic activity occurs with a 3-C chain separating the 2 N's. The side chain amine is best subbed with dimethyls, or other groups of no more than 2-C linear chains. A pyrrolidine counts as 2-C's. Piperazineethanol>piperazine>dimethyl as side chain subs for enhanced activity. |
|
|
Phenothiazine general MTBSM
|
Sulfur will oxidize to sulfoxide, then sulfone. *can happen with air, therefore, these compounds are kept sealed in boddles. Also need to be protected from light and heat, b/c it catalyzes the oxidation.
Oxidative N-dealkylation of sidechain to secondary and primary amine 2'-sub protects one of the phenyl from hydroxylation, the other phenyl will hydroxylate para to N (its more e- rich). |
|
|
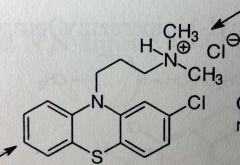
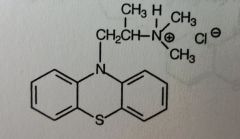
Chlorpromazine HCl. Aliphatic Phenothiazine
Antipsychotic, N/V (antiemetic; most of the anti-dopaminergic agents have this property) |
|
|
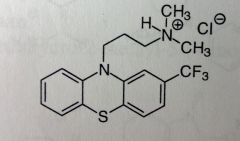
Triflupromazine HCl. Aliphatic Phenothiazine
Antipsychotic, control of severe N/V *F's are very electronegative, strong e- withdrawing grp. Adds some lipophilicty, easier access to BBB. Otherwise very much the same as chlorpromazine. |
|
|
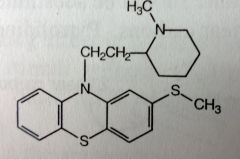
Promethazine. Aliphatic Phenothiazine
N/V, for motion sickness or after general anesthetic use. Also used as an anti-histamine *Doesn't have the e- withdrawing grp on one of the benzenes, also the linker separating the N's is only 2C's---> mostly and anti-emetic agent, b/c these two structural features reduced the antipsychotic activity (10% of chlorpromazine). |
|
|
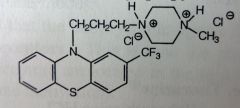
Thioridazine. Piperidine Phenothiazine.
2nd line Schizophrenia Tx (from failure to other Tx). Inhibs post-synap D2, but also inhibitory at α-adrenergic sites. Anti-cholinergic effects aswell. MTBSM: forms 2 active metabolites that use to be marketed aswell. Sulfur ox'd to sulfoxide (mesoridazine)--->more electroneg group--->8x more potent. Ox'd again to sulfone (sulforidazine)-->even more electroneg--->2x more potent than mesoridazine. *Neither is marketed now *Still 3C chain, 2-Sulfur is not that electronegative, but still more than C. |
|
|
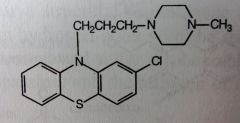
Trifluoperazine HCl. Piperazine Phenothiazine.
Mngmnt of Schizophrenia, short term Tx for non-psychotic anxiety, but not drug of choice). |
|
|
Prochlorperazine. Piperazine Phenothiazine.
Mngmnt of Schizophrenia; Control of N/V related to D2 antagonist activity, antiemetic properties. |
|
|
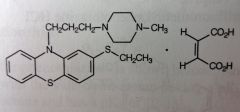
Thiethylperazine Maleate. Piperazine Phenothiazine.
More used for N/V *D/C'd but some generics still available. *note 2-ethyl-sulfide. MTBSM: ox of the ethyl sulfide (-->sulfoxide-->sulfone) leads to active metabolites. |
|
|
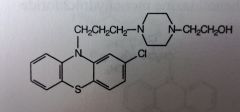
Perphenazine. Piperazineethanol Phenothiazine.
Schizophrenia; severe N/V |
|
|
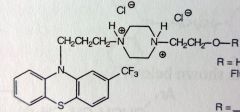
Fluphenazine HCl. Piperazineethanol Phenothiazine.
Schizophrenia and other psychotic disorders. When R=H, t1/2 is only 33hrs. Schizo would have to take it everyday, issue with compliance. Two different fatty acid esters created (7C-Enanthate and 10C-Decanoate). Fatty acid is no longer H2O soluble, vegetable oil suspension for IM depot. Esterase activity in general circulation once drug leaks from depot site, forms Fluphenazine HCl. New t1/2 is 163-232hrs, inj every 1-2 wks, great for compliance. Revolution in anti-psychotic Tx. |
|
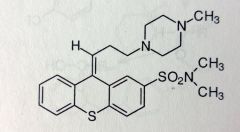
|
Thiothixene. Isoteric Phenothazine Deriv.
Bioisostere: N replaced with C in central ring system. Still similar to phenothiazines in activity (D2-antagonist) and structure: 2-Sulfonamide, electron withdrawing. 3C link to Piperazine. *Z-form is the active isomer |
|
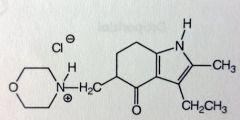
|
Molindone HCl. Indole
Clinically resembles piperazine phenothiazines, used as an antipsychotic for mngmnt of schizophrenia. MoA unknown, could be related to D2-antaginism. Many metabolites, very little leaves unchanged *note morpholine grp, and partially reduced indolone. *probably going to be DC'd soon |
|
|
Butyrophenone SAR
|
4C chain with carbonyl and phenyl (or just two phenyls) on one end=Butyrophenone. The butyrophenone links to Piperadine with an Aromatic sub at 4.
4-F substitution at para position of phenone benzene ring, increases antipsychotic activity. dopamine mimicked by aromatic system off of piperidine at 4'. More potent than phenothiazines, so more CNS SEs, but fewer peripheral Anticholinergic SEs. |
|
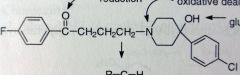
|
Haloperidol. Butyrophenone Anti-psychiotic
PO, IV, Schizophrenia. t1/2= 12-38hrs, taken daily--->compliance issues---> decanotate ester formed at OH---->PRODRUG---> new t1/2 is 3wks---> inj 1xmonth. MTBSM: Reduction of ketone. N-Dealkylation from tertiary amine on piperadine. OH--->gluc'd Two benzenes are protected by F/Cl. ox'd and Conj'd = Deactivation |
|
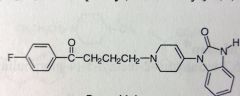
|
Droperidol. Butyrophenone Anti-psychiotic
IV, Antipsychotic, Antiemetic (cancer chemo, surgeries) *removed the OH, now has dbl bond, linked to benzimidazole now, these Ns are not basic, part of urea 3-10 min onset, 2-4 hr DoA, t1/2 = 2 hrs Extensive mtbism dealkylation of middle N |
|
|
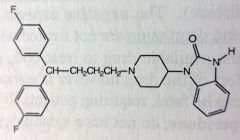
Pimozide. Butyrophenone Anti-psychiotic
Suppression of motor and phonic tics in Tourettes 9by dopamine receptor antagonism) No longer have ketone and lost piperidine dbl bond, t1/2 = 29 hours MTB: phenyls protected by F's. Extensively metabolized like droperidol N-dealkylation |
|
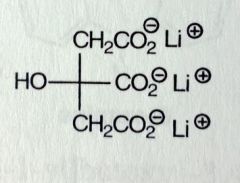
|
Lithium Citrate (organic salt). Misc
Most commonly given as Li-carbonate (inorganic salt) Manic episodes of bipolar disorder, mood stabilizing drug. Inc'd NE reuptake and 5HT receptor sensitivity Ion, so its eliminated through urine *dec'd reabsorption of NA can lead to Na depletion |
|
|
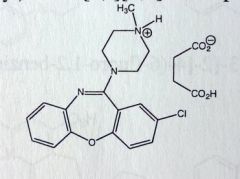
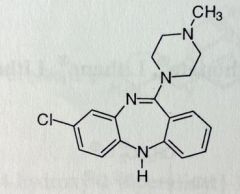
Loxapine Succinate. Misc
Schizophrenia Tx, exact MoA unknown Structurally very similar to TCAs, its amoxapine (TCA) with a methyl. Loxapine can be N-dealkylated to amoxapine. |
|
|
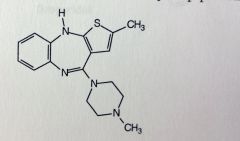
Clozapine. Atypical Anti-psychotics
1st drug to treat pos&neg effects of schizo SE: agranulocytosis, need weekly blood tests Structurally very similar to loxapine. cL moved, O replaced with N. Used only when other drugs ineffective Neg not treated by typicals (withdrawal from society) |
|
|
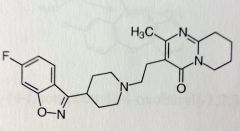
Risperidone. Atypical Anti-psychotics.
PO, IV. Schizophrenia, approved for teenagers DA and 5HT antagonist, more potent DA. Ring system on each end of molecule. The isoxazole has an F subbed Phenyl -> unlikely to be metabolized. Other ring has pyrmidine fused to pyrido, partially reduced. MTB: OH at 9' -> active metabolite (paliperidone) |
|
|
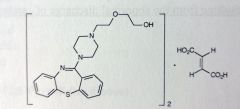
Olanzapine. Atypical Anti-psychotics.
Pos-neg schizophrenia effects. Bipolar disorder. Structurally similar to Clozapine w/o Cl subbed phenyl, one of pnehyls is replaced with thiophene -> bioisotere Activity also related to clozapine but no agranulocytosis. Fewere parkinsons like SEs compared to risperidone. Produces very little sedations. |
|
|
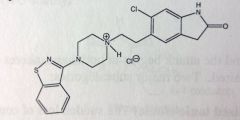
Quetiapine Fumarate. Atypical Anti-psychotics
Schizophrenia and Bipolar disorder. MoA unknown: probably DA and 5HT antagonism. MTB: major metabolites are sulfure oxidation and primary oxidation ones, INACTIVE. MTB similar to phenothiazines Sulfoxide/Sulfone -> inactive Tertiary amine, dealkylation loses hydroxyethyloxy grp -> leaves 2ndary amine -> active metabolite b/c eh? |
|
|
Ziprasidone. Atypical Anti-psychotics
Schizophrenia and Bipolar disorder MoA unknown: Antagonist at DA, 5HT1d, 5HT2a, Agonist at 5HT1a 2 aromatic ring systems with piperazine and tertiary amine containing linker. MTB: Mostly Phase 1 or rxns results in inactivation. Sulfur of isothiazole -> sulfoxide/sulfone -> inactive |
|
|
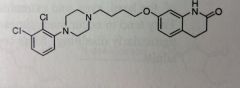
Aripiprazole. Atypical Anti-psychotics.
Schizophrenia and Bipolar disorder. MoA unknown: partial agonist at D2 and 5HT1a antagonist at 5HT2A Two aromatic systems with piperazine containing linker. Highly BA, orally 87% MTB: Most important is the dehydration forming a double bond |
|
|
EPILEPSY
|
Chronic seizures
- Can have partial seizures: only one hemisphere, last 1-1.5 minutes, w/ or w/o loss of consciousness - Or generalized seizures: both hemispheres, will get loss of consciousness. o Two types of generalized: grand mal or absence (or petit mal) Grand mal: sudden loss of consciousness and general muscle spasms lasting 2-5 min (more common in children) Petit mal: brief loss of consciousness (5-30 sec), can occur many times - Status epilepticus: more serious, prolonged seizures, 30mins, or repeated seizures with not enough time between for patient to recover. Can be fatal if not treated properly Mechanism of seizures: believed to be related to GABA system. Enhancing activity of GABA can improve epilepsy. Increase GABA in CNS: increase its synthesis, inhibit its elimination, potentiate action of GABA at its receptor (like with barbiturates and benzos), or inhibit excitatory NTs (decrease glutamate concentration or decrease binding to glutamate receptors) |
|
|
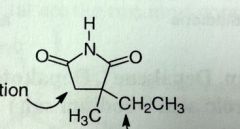
Ethosuximide. Succinimide.
Petit mal MoA: Depresses motor cortex in CNS and elevates threshold of convulsive stimuli (can tolerate higher amt w/o causing seizure) Not good drugs, SEs, and problems |
|
|
Drugs for petit mal seizures (absence, type of generalized)
|
Succinimides, oxazolidinediones, valproic acid, acetazolamide, carbonic anhydrase inhibs, clonazepam.
|
|
|
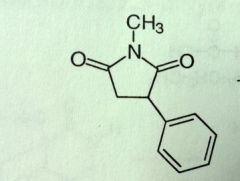
Phensuximide. Succinimide.
Petit Mal Phenyl sub, methyl on N d/c'd |
|
|
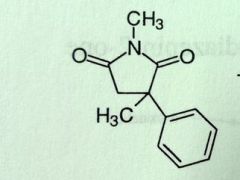
Methsuximide. Succunimide.
Petit mal. Phenyl and methyl sub, methyl on N t1/2 of parent: 4-6 hours, very short, demethylated metabolite, t1/2 = 25 hours, metabolite responsible for most of activity MTB: methyl introduced on N demethylation rxn (phensuximide and methsuximide) forms active |
|
|
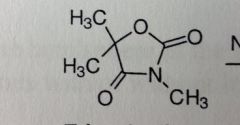
Trimethadione. Oxazolidinedione.
Petit mal (only when other less toxic drugs are ineffective). SEs: Very toxic to skin and blood (lupus, myasthenia gravis) PRODRUG: activated through N-demeth T1/2 paretn = 16-24. After activation, t1/2 = 6-13 DAYS |
|
|
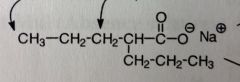
Valproic acid.
*fatty acid, two propyl groups linked to acetic acid (either Na or mixture of Na/acid) Moa unknown, probably potentiation ofGABA SEs: Liver tox, pancreatitis, teratogenic MTB: Ox'd at aliphatic sidechains, omega/beta possible Omega will continue to Ox until COOH (now its 2-propyl) |
|
|
Acetazolamide.
Inhibits carbonic anhydrase (suppose to catlyse interconversion of CO2 to Carbonic acid, depending on site of action. Eye: carbonic anhydrase -> dec carbonic acid formation -> dec secretion -> tx for glaucoma b/c dec'd intraocular pressure Acute mountain sickness petit mal Kidney: inhib conversion to CO2 (mild diuretic properties) |
|
|
Clonazepam. BZDs.
petit mal. The other BZDs have anticonvlusant properties used as adjunct Tx for many types of seizures. Often used in combo with other agents for control of stat epilepticus. MTB: nitro reduced, otherwise same as BZDs *nitro |
|
|
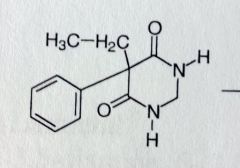
Pyrimidone
Weak anticonvulsant activity, good for grand mal MoA unknown b/c yo uremoved c=o, drug is not acidic, the H's on the nitrogens no longer acidic. Amides are neutral. *bioisotere of the grand mal barbs. Missing C=O but maintains activity |
|
|
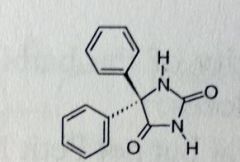
Phenytoin Sodium. Hydantoins.
Grand mal Hydantoins: 5 membered rings, similar to barbs. Work in the CNS at Motor Complex, tries to prevent spread of Grand Mall and Partial seizures by promoting NA efflux, thereby hyperpolarizing. NH in between the two C=Os is the acididc one t1/2 = 22 hrs |
|
|
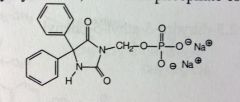
Fosphenytoin. PRODRUG. Hydantoins.
IV b/c phos is highly soluble. for Status epilepticus and seizures occurring during neurosurgeries. IV allows quick admin to these dangerous conditions. Phos'd at the acidic N of phenytoin can be cleaved by phosphatases at sites of action. Gives release of hydroxymethyl succinamide intermediate, needs to be demethylated. |
|
|
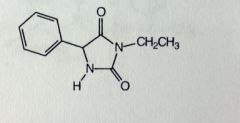
Ethotoin. Hydantoins.
Grand Mal MTB: similar to others. OH'd at phenyl N-dealkylation, ethyl removed. *ethyl sub, with one less phenyl |
|
|
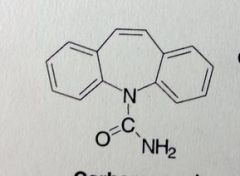
Carbamazepine. Dibenzazepines.
Grand Mal, Bipolar Disorder (so its also an atnipsychotic as effective as Li+) Carbamyl grp attached to N on central ring system, urea, neutral grp. MTB: the 10'-11' dbl bond will convert to an epoxide through cyp3a4. The epoxide is more stable here than on benzene ring systems. Will quickly rearrange to give diol. |
|
|
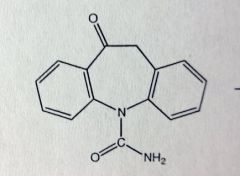
Oxcarbazepine. Dibenzazepines.
Partial seizures. *PRODRUG of active hydroxy-metabolite (Licarbazepine). Epoxide can convert to hydroxy through a rearrangement reaction. If the hydroxy is ox'd, it'll form the ketone prodrug. MTBSM: Ketone reduced to active drug; want S-enantiomer Prodrug with cleavable acetate ester available soon. |
|
|
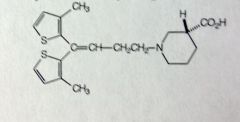
Tiagabine
partial seizures MoA: inhibits GABA reuptake, potentiates GABA effects MTBSM: extensive only 2% left unchanged Gluc'd at COOH Thiophenes are OX'd. electron rich. Starts with oxidation next to sulfur, then hydroxylation, which can tautomer to form =O. Inactive once thiophene is ox'd. *carboxylic acid, 2 metyls thiophene SE: Conc 40% lower when taken at night (Diurnal effect), more elim at night |
|
|
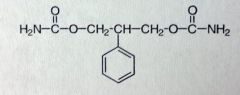
Felbamate
Partial seizures only when other therapy fails. Moa unknown SEs: Aplastic anemia, acute liver failure Structure is similar to Miltown but not used for anxiety, Changed center methyl/propyl subs to phenyl/h |
|
|
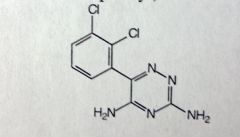
Lamotrigine
partial seizures MoA unknown: could be related to decreased voltage of Na channels MTBSM: 70% eliminated by gluc'd at 3' amino *triazine, dichlorophenyl, two amino grps |
|
|
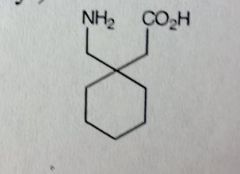
Gabapentine
Partial Seizures MoA unknown *Designed as cyclohexyl analog of GABA. Idea was to mimic both effects and reuptake, but found no relationship to GABA receptor binding or reuptake inhibition. Does produce GABA like activity |
|
|
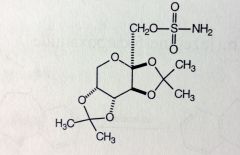
Topiramate
Partial Seizures, Grand Mal MoA unknown, likely blocks spread of seizure by Na+ channel hyperpolarization MTBSM: not extensively metabolized 70% unchanged *a protected sugar, the OHs are protected by two acetones and sulfonamide ester |
|
|
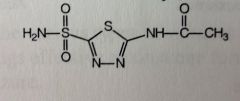
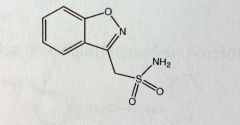
Zonisamide
Partial seizure adjunctive Tx *methyl-sulfonamide attached to benz-isoxazole system MTBSM: Acetylation of sulfonamide Can cleave isoxazole between O-N Gluc'd at sulfone 60-70% MTBSised |
|
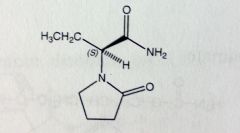
|
Levetiracetam
Partial seizures. Given as S-enantiomer. MoA unknown. *5-membered pyrrolidine-1 system attached to Acetamide MTBSM: about 1/4 of dose inactivated by oxidation of primary amide to COOH |
|
|
MgSO4
|
Magnesium Sulfate
IV Tx of choice for prevention of Eclampsia (acute and life threatening complications of pregnancy, seizures, and coma) Is CNS depressant. Mg can block neuromuscular transmission and decrease amount of ACh liberated to motor endplate |

