![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
84 Cards in this Set
- Front
- Back
|
What % of adults infected with HIV-1 become infected through the exposure of mucosal surfaces vs. percutaneous or IV routes?
|
Mucosal transmission - 80%
PerQ/IV - 20% |
|
|
What is the minimal amount of virus needed to be detected in plasma by PCR vs. quantitative clinical assays used to monitor viral load.
|
Immediately after exposure and transmission, as HIV-1 is replicating in the mucosa, submucosa, and draining lyphoreticular tissues, the virus ccannot be detected in plasma - this "eclipse phase" lasts 7-21 days.
At 1-5 copies per mL in plasma, the virus can be detected with the use of sensitive qualitative methods of nucleic acid amplification. At concentrations of 50 copies per mL, HIV-1 can be detected by means of quantitative clinical assays used to monitor viral load. |
|
|
The progression of HIV-1 infection can be depicted as 6 distinct stages. What are the distinct stages?
|
I (0-18 days): Eclipse - viral replication occurs. Viral RNA +
II (18-21 days): Gag p24 Ag +, inflammatory cytokines, CD8 T cell responses, acute phase reactants, viral latency. III (23-25 days): ELISA +, Antibody-Antigen complexes IV (25-31 days): gp41 antibodies V (1-3 months): Western blot +, p31 - VI (>3 months): Western blot +, p31+ |
|
|
What are the first cell-type targets of HIV?
|
CD4 T cells and Langerhans' cells are the first targets of HIV
Other dendritic cells also may play an important accessory role. Regardless of the route of transmission and the first cells infected, within a few days, viral replication converges on the lymphoreticular system of the gastrointestinal tract (i.e., gut-associated lymphoid tissue). In this tissue, in both humans and macaques, the phenotype of most productively infected cells appears to be the resting CD4 T cell lacking activation markers and expressing low levels of the chemokine receptor CCr5. Many of these cells express alpha4beta7 integrin receptors and Type 17 helper T cell surface markers. Since these receptors are also detected on T cells harvested from the genital mucosa, they may play an important role in HIV acquisition. The rapid expansion of HIV-1, first in gut-associated lymphoid tissue and then systemically, along with a a sharp rise in plasma levels of viral RNA, is clinically important b/c of the coincident irreversible destruction of reservoirs of helper T cells and the establishment of viral latency (defined as the silent integration of HIV-1 DNA into the genomes of resting T cells, an effect that has stymied curative treatment efforts. |
|
|
A single virion is responsible for HIV-1 transmission in approximately ___% of heterosexuals, ____% of men who have sex with men, and ____% of injection drug users.
|
80% of heterosexuals
60% of homosexual men 40% of IVDA (in IVDAs, as many as 16 transmitted virions have been found to be responsive for productive infection, which would be consistent with the absence of a mucosal barrier to transmission. The phenotypes of cloned proviruses corresponding to transmitted (or founder) viruses are nearly always CD4 and CCR5 T-cell tropic variants and exhibit neutralization-sensitivity patterns that are typical of primary viral strains. These phenotypic properties are present at the moment of transmission, when the virus encounters the first target cell; they are not the conequence of viral adaptation to the new host. |
|
|
What is the initial innate immune response to HIV-1?
|
The first signal of an immune response to HIV-1 infection is the appearance of acute-phase reactants, including alpha-1-antitrypsin, and serum amyloid A, in plasma 3-5 days after transission.
The steep rise in HIV-1 viral load (ramp-up viremia) coincides with a large burst of inflammatory cytokines led by interferon-alpha and interleukin-15 and a shower of plasma microparticles with surface phophatidylserine, derived from infected and activated CD4 T cells undergoing apoptosis; these particles have immunosuppressive properties. The earliest cytokines are produced by dendritic cells, but alter in the infective process, multiple cell types (e.g., monocytes, macrophages, NK cells, and T cells) also produce these mediators. Although cytokines enhance protective antiviral immune responses in acute HIV-1 infection, the cytokine storm probably also contributes to harmful immune activations and loss of CD4 T cells. NK cells are activated in acute HIV-1 infection and, in vitro, kill cells infected wtih the virus. NK cells have a range of receptors that either enhance or inhibit their function. NK-cell immunoglobulin-like receptors interact with HLA molecules with soem specificity for the peptides thay bind. This activity might explain the genetic associations b/w certain NK-cell Ig-like receptors and HLA types with more favorable otucomes of infection. |
|
|
Describe the adaptive immune responses in acute HIV-1 infection
|
The initial antibody response to the viral envelope is non-neutralizing and does not secelt for viral escape. Antibodies that neutralize the transmitted founder virus are not detected until 3 months or more after infection.
Although many of the targets of neutralizing antibodies are on the glycoprotein-120 component of the HIV-1 envelope, the initial antibody response to HIV-1 is focused on non-neutralizing sites of the glycyoprotein 41 envelope stalk. by the time a potentially effective antibody response has developed, it is much too late to influence the course of the infection. The first CD8 T-cell responses appear days before the peak of viremia and focuse on b/w 1 and 3 distinct epitopes - most commonly nef and gag. |
|
|
The per-person probability of transmitting HIV-1 is most closely correlated with teh viral burden in blood, each time the viral burden icnreased by a factor of 10, the risk of transmission is expected to increase by a factor of ___.
|
2.5
|
|
|
What are medical prevention strategies to prevent HIV?
|
Offering antiretroviral agents to people at risk before or immediately after HIV exposure or as a menas of secondary prevention. Use of the antiretroviral drug tenofovir as a topical prophylactic agent before viral expure in women at high risk led to a 39% reduction in incident cases of HIV inection that was directly correlated with concentrations of the drug in mucosal tissue.
|
|
|
How quickly after exposure can HIV infection be detected?
|
Immediately after exposure and transmission, as HIV-1 is replicating in the mucosa, submucosa, and draining lymphoreticular tissues, the virus cannot be detected in plasma; this so-called eclipse phase generally lasts 7 to 21 days.
The stages that define acute and early HIV-1 infection are characterized by the sequential appearance of viral markers and antibodies in the blood. More sensitive, fourth-generation tests, which detect both antigens and antibodies, shrink the virus-positive–antibody-negative window by about 5 days. Testing for viral RNA in plasma closes this gap by an additional 7 days. |
|
|
When are patients infected with HIV most contagious?
|
The per-person probability of transmitting HIV-1 is most closely correlated with the viral burden in blood; each time the viral burden in an HIV-1–infected person increases by a factor of 10, the risk of transmission is expected to increase by a factor of 2.5.
The risk of contagion from patients with acute, early infection appears to be much higher than that from patients with established infection, at least in part because of the high viral load and the homogeneity of viral variants clearly capable of causing infection. |
|
|
What is the efficacy of preexposure prophylaxis for HIV?
|
Use of the antiretroviral drug tenofovir as a topical prophylactic agent before viral exposure in women at high risk led to a 39% reduction in incident cases of HIV infection that was directly correlated with concentrations of the drug in mucosal tissue. A multinational trial focused on men who have sex with men showed that a once-daily pill containing tenofovir plus emtricitabine provided an average of 44% protection over and above that conferred by the provision of comprehensive preventive services, including provision of condoms and counseling. The level of protection varied widely, depending on how consistently participants used preexposure prophylaxis.
|
|
|
Which antiretrovirals are likely to be most effective for the treatment of acute HIV?
|
If antiretroviral therapy is to be provided for patients with acute infection, the preferred treatment regimen, which has yet to be determined, might include antiviral agents that concentrate in the genital tract of men and women and an integrase inhibitor, the latter because of the rapidity with which this class of agents lowers the viral load.
|
|
|
What are the symptoms of acute HIV infection?
|
The acute retroviral syndrome occurs 2 to 3 weeks after infection, usually coinciding with the onset of viremia, and is accompanied by several nonspecific symptoms, including fatigue, pharyngitis, weight loss, myalgias, and headache.
|
|
|
What are the diagnostic hallmarks of acute HIV infection?
|
The diagnostic hallmarks of acute HIV infection are a negative test for HIV-1 antibodies on enzyme immunoassay (EIA), an indeterminate or negative test for HIV-1 on Western blot analysis, and detection of HIV RNA in plasma.
|
|
|
Guidelines suggest treatment for which patients with HIV infection?
|
Currently, guidelines for most developed countries advocate treating asymptomatic HIV infection in patients who have a CD4 T-cell count below 500 cells per cubic millimeter, with many suggesting treatment at higher CD4 T-cell counts. World Health Organization guidelines take a more conservative approach, suggesting treatment at the threshold CD4 T-cell count of 350 cells per cubic millimeter for asymptomatic patients unless they are pregnant. South African guidelines recommend treatment for healthy patients with HIV infection who are not pregnant and have a CD4 count of 200 cells or less per cubic millimeter, due to the perceived costs and burden on available services.
|
|
|
A 30 year-old female presents with an acute febrile illness. Her partner is HIV-infected and she had a recent HIV antibody test, which was negative. She reports that they been sexually active and have not been using condoms consistently. You send an HIV antibody test and HIV RNA level.
Which one of the following test results would be consistent with acute HIV infection? a). HIV ELISA positive, HIV RNA <400 copies/ml. b). HIV ELISA negative, HIV RNA >750,000 copies/ml c). HIV ELISA positive, HIV RNA 100,000 copies/ml. d). HIV ELISA negative, HIV RNA 1,000 copies/ml |
Answer B
During the acute phase of HIV infection, the HIV viral load is very high and antibody is negative. |
|
|
You are seeing a 30-year-old male who was recently hospitalized with cryptococcal meningitis and was found to be HIV-infected during this admission. He was treated with amphotericin and discharged on fluconazole and trimethoprim-sulfamethaxazole (TMP-SMX). His CD4 count was 8 and viral load 634,000. Toxoplasma and CMV IgG were positive. He has a history of TB exposure a few years ago and was treated with INH for 9 months at the time.
Which ONE of the following medications should also be prescribed at this time? a). Dapsone 100 mg daily b). Valgancyclovir 450 mg twice daily c). Isoniazid 300 mg daily d). Azithromycin 1200 mg weekly |
Answer D - Azithromycin
Prophylaxis for Mycobacterium avium is indicated when the CD4 count is below 50 cells/mm3. Dapsone can be used for Pneumocystis prophylaxis, but this patient is already on trimethoprim-sulfamethaxozole, so this would not be needed. Valgancyclovir can be used to treat CMV retinitis and for secondary prophylaxis when a patient has a history of CMV retinitis, but is not recommended for primary prophylaxis (however, this patient should have retinal exam to look for CMV retinitis). Isoniazid would not be indicated, since he has already been treated for tuberculosis before. |
|
|
A 55-year-old male with HIV infection comes for routine 3-month follow-up. His most recent CD4 counts were 320/ml and 340/ml, viral loads 56,000 copies/ml and 48,000 copies/ml respectively. He has a history of stable angina, but feels well and has no complaints. He works as a store manager and is in a long-term relationship with a male partner who is also HIV-infected. He says he is ready to begin antiretroviral therapy if you feel it is best.
Which ONE of the following would be the best option? a). Start antiretroviral therapy when his CD4 count falls below 300. b). Start antiretroviral therapy now. c). Only start antiretroviral therapy if the CD4 cell count is declining. d). Start therapy if the viral load is over 100,000. |
Answer B - start antiretrovirals now
He is at a stage where he should be on antiretroviral therapy. The decision to start therapy is based on the CD4 count; guidelines would recommend initiation of therapy when CD4 count declines below 500, particularly when they are below 350. The viral load and short-term trends in the CD4 count are not used to determine whether antiretroviral therapy is indicated. |
|
|
A 46-year-old male is found to be HIV-infected on routine screening. His CD4 count is 256 and HIV viral load 96,000 copies/ml. He is ready to start therapy. According to current guidelines, which of the following should be prescribed?
a). Prescribe a two-drug combination of two nucleoside reverse transcriptase inhibitors (NRTIs) and add a non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI) if the viral load is still detectable after three months. b). Prescribe a three-drug combination of two nucleoside reverse transcriptase inhibitors (NRTIs) with a non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI). c). Prescribe a four-drug combination of two nucleoside reverse transcriptase inhibitors (NRTIs) with a non-nucleoside reverse transcriptase inhibitor (NNRTI) and a protease inhibitor (PI); then stop one of the four once the viral load is suppressed. d). Prescribe a three-drug combination of two nucleoside reverse transcriptase inhibitors (NRTIs) with a protease inhibitor (PI); boost the protease inhibitor with ritonavir if the viral load is not suppressed after three months. |
Answer B - 3 drug combo of 2 NRTIs + NNRTI or PI if viral load is still detectable after 3 months
Current guidelines recommend initiating treatment with a three-drug combination of two nucleoside reverse transcriptase inhibitors (NRTIs) with one of the following: a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI), or an integrase inhibitor. Protease inhibitors should be boosted with ritonavir. |
|
|
A 42 year old male with longstanding HIV infection is transferred to your care. His other medical problems include hepatitis C infection from prior cocaine use. He has been smoking since the age of 13 and currently smokes ½ pack of cigarettes daily. His CD4 count is 422 cells/mm3 and he has a HIV-1 viral load of 55,000 copies/ml. His baseline serum creatinine is 1.5 mg/dL. His recent lipid values show total cholesterol of 199 mg/dL, HDL of 45 mg/dL and LDL of 90 mg/dL. A screening test for hepatitis B is negative.
In choosing a starting regimen for this patient, several considerations need to be made, including dosing schedule, side effects, and drug/drug interactions. In the case above, which of the following is the most important factor in choosing an NRTI "backbone"? a). Tenofovir and emtricitabine can be given once daily. b). Tenofovir and emtricitabine increase the risk of a cardiovascular event. c). Tenofovir and emtricitabine can decrease his GFR. d). Tenofovir and emtricitabine are active against Hepatitis |
Answer C - Tenofovir and Emtricitabine can decrease GFR
Although tenofovir is on the preferred list of drugs for the selection of NRTIs, it is associated with decreases in GFR and tubular dysfunction. In the setting of pre-existing kidney disease, alternative NRTIs are preferred over dose-adjusted tenofovir. |
|
|
A 39-year-old male with HIV infection is on a regimen of two NRTIs in the form of Combivir (lamivudine and zidovudine) with the protease inhibitor Kaletra (lopinavir/ritonavir). He is doing well and has had consistently undetectable viral load with rising CD4 count. His fasting lipid panel shows total cholesterol of 269mg/dl with an HDL of 40mg/dl and LDL of 179mg/dl and triglycerides 250mg/dl. He smokes cigarettes, but has no other risk factors for coronary artery disease.
Which ONE of the following would be the best course of action for this patient? a). Prescribe simvastatin 40 mg daily. b). Replace Combivir with Truvada (tenofovir and emtricitabine) c). Prescribe pravastatin 40 mg daily d). Replace Kaletra with ritonavir-boosted fosamprena |
Answer C - prescribe pravastatin 40 mg daily
Hyperlipidemia is associated with most of the protease inhibitors, including lopinavir/ritonavir. Replacing this combination with ritonavir-boosted fosamprenavir, or replacing the NRTIs would not help. Prescribing pravastatin would be appropriate; simvastatin should not be prescribed because its metabolism is inhibited by ritonavir and this can lead to toxicity. |
|
|
A 35-year-old male who injects heroin and cocaine comes to the Emergency Department complaining of fever, sore throat and malaise. He has a history of hepatitis C and admits that he has recently shared needles with others. A rapid oral HIV test is negative. He has two sets of blood cultures drawn and is started on antibiotics.
Which ONE of the following tests should be ordered next? a). CD4 count b). Hepatitis C RNA level c). Serum HIV antibody and Western blot d). HIV RNA level (viral load) |
The correct answer is D: HIV RNA level (viral load).
|
|
|
HIV preferentially infects human T lymphocytes of the helper/inducer subset (also called CD4 cells). The HIV life cycle is divided into six steps, which are?
|
1. Binding and fusion. HIV binds to CD4 receptors and co-receptors, then fuses with and releases its genetic material into the host cell.
2. Reverse transcription. Reverse transcriptase converts the single-stranded RNA of the virus to a double-stranded HIV DNA. 3. Integration. The HIV DNA enters the host cell's nucleus and an HIV enzyme, integrase, integrates the HIV DNA into the host cell DNA. This 'provirus' can remain dormant for years before creating new copies. 4. Transcription. When the host cell is activated, the host's RNA polymerase creates copies of the HIV genomic material, as well as mRNA, which is used to make HIV proteins. 5. Assembly. Protease, an HIV enzyme, cuts the chains of HIV proteins and these smaller proteins join with HIV RNA to create new virus particles. 6. Budding. The newly-assembled virus 'buds' from the host cell, taking part of the host cell's outer membrane in the |
|
|
The mean length of time from seroconversion to symptomatic AIDS in untreated patients is ______ years; however, this varies considerably from person to person and probably reflects variations in individual immune systems and the virulence of different strains of the virus. Expanded use of antiretroviral therapy, prophylaxis against opportunistic infections, and treatment of AIDS-associated conditions has resulted in delayed progression and longer life expectancies for HIV-infected people
|
The mean length of time from seroconversion to symptomatic AIDS in untreated patients is 10 years; however, this varies considerably from person to person and probably reflects variations in individual immune systems and the virulence of different strains of the virus. Expanded use of antiretroviral therapy, prophylaxis against opportunistic infections, and treatment of AIDS-associated conditions has resulted in delayed progression and longer life expectancies for HIV-infected people
|
|
|
How can you diagnose acute HIV infection?
|
Because of the nonspecific nature of the illness, practitioners need to maintain a high index of suspicion to identify patients with primary HIV infection. The diagnosis can be made by measuring HIV RNA level (viral load); an elevated viral load (usually over 100,000 copies/mL) in the presence of a negative HIV ELISA, establishes the diagnosis. Viral load is 100% sensitive and 97% specific; however, specificity for viral load approaches 100% if a cutoff of 10,000 copies/mL is used (i.e. false-positive viral load titers are usually <10,000 copies.
|
|
|
Why do we measure an CD4 count in HIV/AIDS?
|
The absolute number and percentage of CD4 lymphocytes gives the clinician an indication of the degree of immunosuppression, and can be used to determine those complications the patient is susceptible to.
The absolute number of CD4 cells is subject to more variability than the percentage because of normal biologic fluctuations in total lymphocyte counts. Within a year of seroconversion, CD4 cell counts usually drop 200 to 300/mm3 from the normal range of 800 to 1200. This is followed by a slow decline of less than 100 cells per year. People with CD4 cell counts greater than 500 are usually asymptomatic and have virtually no risk of developing an AIDS-indicator condition within 18 months, except for TB, cervical cancer, recurrent bacterial pneumonias, or superficial Kaposi sarcoma (KS). In contrast, those with CD4 cell counts of 100 or less are often symptomatic and have a 60% chance of developing an AIDS-indicator disease within 18 months. |
|
|
What is the significant of measuring the viral load in HIV/AIDS?
|
1) Diagnosis of acute HIV, esp. if the ELISA is negative
2) Can be used to monitor the effectiveness of antiretroviral therapy 3) On average, higher viral loads are associated with a more rapid fall in CD4 counts and clinical disease progression...but it has limited prognostic value for an individual patient. |
|
|
A 60-year-old woman is found to be HIV-infected in the course of an evaluation for unexplained weight loss. Her CD4 count is 86 cells/mm3 and HIV RNA level is 526,790. Toxoplasma IgG is negative and CMV IgG is positive. Hepatitis B surface antigen is positive, surface antibody is negative.
Which ONE of the following preventive health care measures would be indicated at this time? a). Prescribe Valgancyclovir 900 mg daily. b). Prescribe Azithromycin 1200 mg weekly. c). Prescribe TMP/SMX daily. d). Begin Hepatitis B vaccination series |
Answer C - begin PCP prophylaxis if CD4 count <200.
|
|
|
Vaccination recommendations in HIV patients
|
Preventive care for HIV-infected patients should include various vaccinations.
Pneumococcal pneumonia is the leading cause of bacterial pneumonia in HIV-infected patients; pneumococcal vaccine is recommended for all HIV-infected patients and is generally repeated every 5 years. The evidence for the efficacy of the pneumococcal vaccine is mixed; a randomized controlled trial in Uganda unexpectedly showed a trend toward increased pneumococcal disease among vaccine recipients, whereas an analysis of a U.S. database of HIV-infected patients suggested a benefit for those with CD4 counts over 500. The influenza vaccine has been shown to be effective and safe in HIV-infected adults; however, the intranasal influenza vaccine (Flumist) should not be given to HIV-infected individuals, since it contains live-attenuated virus. Hepatitis B vaccine should be given in hepatitis B surface antigen and antibody-negative patients because of the increased risk for chronic hepatitis in HIV-infected patients. Hepatitis A vaccination is also recommended for those who are not already immune. Although data on the efficacy of other killed or inactivated vaccines is not available, it is still recommended that routine vaccines such as tetanus be given to HIV-infected patients |
|
|
Antimicrobial prophylaxis in HIV/AIDS.
Comment on: - PCP PPx - TB PPx - Toxoplasmosis - MAC - CMV Retinitis |
Pneumocystis jiroveci pneumonia (PCP) prophylaxis should be initiated when the CD4 cell count is less than 200, or if the patient already has had PCP or has oral candidiasis. Oral trimethoprim-sulfamethoxazole (TMP/SMX) is the preferred agent because it has been shown to be most efficacious and it also has the added benefit of being inexpensive (less than $50 for a year of treatment). If patient cannot take TMP/SMX due to side effects, dapsone, aerosolized pentamidine, or atovaquone can be substituted.
All HIV-infected patients with positive tuberculin tests (defined as ≥5 mm of induration) or close contact with someone with infectious TB, should be treated prophylactically with isoniazid 300 mg and with pyridoxine 50 mg daily for 9 months. If the patient is known to have been exposed to drug-resistant TB, public health authorities should be consulted. Patients with antibodies to Toxoplasma gondii and CD4 cell counts less than 100 cells/mm3 should be given TMP/SMX (one double-strength dose per day) for prophylaxis. Dapsone and pyrimethamine are acceptable alternatives if TMP/SMX is not tolerated. Prophylaxis is recommended against Mycobacterium avium complex (MAC) when CD4 cell counts are less than 50 cells/mm3 and has been shown to reduce the risk of disseminated infection and overall mortality. Effective regimens include azithromycin 1200 mg once a week, clarithromycin 500 mg twice daily, or rifabutin 300 mg daily. Azithromycin and clarithromycin are more efficacious than rifabutin, and azithromycin has the added advantage of once weekly dosing. Since the advent of new antiretroviral agents that can raise CD4 cell counts for prolonged periods, there has been increasing evidence that primary (and secondary) prophylaxis can be discontinued once the CD4 cell count rises above the value used as the threshold for initiation. Discontinuation of prophylaxis for P. jiroveci, T. gondii, and MAC has been shown to be safe when patients have a sustained increase in CD4 counts above the prophylaxis threshold CMV Retinitis - retinal exam every 6 months if CD4 count <50 |
|
|
A 42-year-old male was recently diagnosed with HIV infection and comes to see you for the first time. His CD4 count was 320 cells/ml and HIV viral load 67,000 copies/ml. He feels well and has no physical complaints. He is on no prescribed medications.
Which one of the following would be the best next step in the management of this patient? a). Assess his readiness to take medications for his HIV infection. b). Prescribe trimethoprim-sulfamethoxazole daily. c). Have him return in 3 months for a repeat CD4 count and HIV viral load. d). Prescribe antiretroviral therapy. |
Answer A - assess his readiness to take life-long meds for HIV infection
|
|
|
Describe the basic indications of starting antiretroviral therapy in patients with HIV/AIDS.
What are Clinical Conditions Favoring Initiation of Therapy Regardless of CD4 Cell Count? |
There is an ongoing debate about the optimal timing of antiretroviral therapy. There is a consensus that everyone with an AIDS-defining illness or a CD4 count below 350 cells/mm3 should be offered treatment.
There is growing observational data that indicates that earlier treatment is associated with improved survival. As a result, many experts and guidelines recommend treatment at a CD4 count below 500 cells/mm3. Some experts feel that everyone with HIV should be treated, regardless of CD4 count. It is important to keep in mind that initiating treatment is a lifelong commitment and that patients should be prepared for this. Conditions favoring initiation of therapy regardless of CD4 count: - pregnancy (to prevent transmission to the fetus at the time of delivery) - HIV-associated nephropathy (which has been shown to respond to anti-retroviral therapy). - Hx of or current AIDS-defining illness - CD4 decline of >100 per year - HIV RNA level of >100,000 copies/mL - Hepatitis B infection (when treatment for hepatitis B is indicated) since a number of agents used to treat hepatitis B infection also have activity against HIV (including lamivudine and adefovir) and when given alone, may result in the HIV becoming resistant to those agents |
|
|
Ms. Connelly is a 29-year-old woman recently diagnosed with HIV after presenting with zoster. She has three children and reports a pattern of inconsistent condom usage with her current partner, whom she believes gave her HIV. She has a prior history of genital herpes with one to two outbreaks per year. She is not currently on any birth control or other medications. She does not smoke, drink alcohol or have a history of injection drug use. Physical examination is unremarkable.
Her labs reveal a CD4 count of 440 cells/mm3 and a viral load of 52,000 copies/mL. Urine hCG is negative. Genotypic resistance testing reveals no mutations. She has normal renal function and you decide to start her on a fixed combination of tenofovir and emtricitabine paired with either efavirenz or a boosted protease inhibitor. Which of the following would be the most important reason to select a boosted PI over efavirenz? a). Antiretroviral potency b). Side effect tolerability c). Once-daily dosing d). Desire for pregnancy |
Answer D - Efavirenz is teratogenic
|
|
|
When would you consider using CCR5 inhibitors or Fusion Inhibitors in HIV/AIDS?
|
These are reserved for "salvage therapy" if there is resistance to the first-line therapies.
|
|
|
The decision of which combination of antiretroviral medications should be used for HIV/AIDS is a complicated one and should probably be only made by an experienced practitioner. There are a few general principles that are helpful to keep in mind, which include:
|
- Three drugs are generally used for initial therapy, although four or more may be used if patients have an inadequate response to standard three-drug regimens.
- Initial therapy generally includes a "backbone" of two NRTI's plus one of the following: a NNRTI, a PI or an Integrase inhibitor. - Protease inhibitors are generally boosted with ritonavir, which inhibits the cytochrome P450 enzyme CYP3A4 and allows for lower and less frequent dosing of other PIs. Ritonavir also interacts with many other medications. - Some medications need to be dose-adjusted for renal function; this includes all of the NRTI's, except for abacavir. - Choices of regimens are often influenced by the availability of formulations that contain two or three medications in a single pill, this allows for simplified dosing and lower pill burden. The simplest regimen is a once-daily single-pill combination of efavirenz, tenofovir and emtricitabine, which is marketed as Atripla. - Protease inhibitor-based regimens are associated with GI side effects and lipid abnormalities, while the NNRTI efavirenz is associated with rash and CNS side effects. - Efavirenz should not be used during the first trimester of pregnancy or in women trying to conceive or not using effective birth control. |
|
|
Suggested steps in the process of choosing an antiretroviral regimen
|
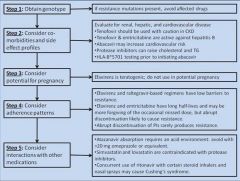
|
|
|
A 37-year-old female is diagnosed with HIV infection during a hospitalization for pneumocystis pneumonia. Her initial CD4 count was 66 cells/mm3 and viral load 711,000 copies/ml. An HIV genotype done at the time of diagnosis shows no mutations associated with drug resistance. After initial treatment of the pneumonia, she is started on efavirenz, tenofovir and emtricitabine in the single-tablet daily formulation (brand name Atripla). A month after initiation of therapy, her CD4 has risen to 127 cells/mm3 and the viral load has fallen to 31,000 copies/ml. Three months after initiation of therapy, her CD4 count is 176 cells/mm3 and viral load is 5,600 copies/ml. She reports that she did run out of her medication for a few days after the first month of treatment, but has not missed any doses in the past 2 months.
What is the best next step in the treatment of her HIV infection? a). Continue Atripla at current dose (one tablet daily) b). Increase Atripla to two tablets daily. c). Check a HIV genotype for resistance mutations. d). Add raltegravir to the regimen. |
Answer C - Check HIV genotype for resistance. After 3 months, a persistent viral load is concerning for resistance.
|
|
|
After initiating antiretroviral therapy, how often should viral load be monitored?
|
When antiretroviral therapy is initiated or changed, the viral load should be monitored 2 to 4 weeks later and then at 3-month intervals to assess continued efficacy. A 1.0 log decrease in viral load (e.g., from the hundreds of thousands to tens of thousands) should be achieved after 2 to 4 weeks of therapy. After 12-24 weeks of therapy, the viral load should be below the level of detection (fewer than 50 copies/ml with ultrasensitive tests).
|
|
|
Drug resistance and antiretroviral therapy.
- What is the best way to prevent development of resistance? - When is resistance testing most useful? |
The best way to prevent development of resistance is to maximally suppress viral replication and to maintain viral suppression. The only other way to prevent the development of resistance is to not expose the patient to antiretroviral medications in the first place (particularly if the patient is not ready for the commitment that antiretroviral therapy requires). A mutation that confers resistance to a specific antiretroviral agent may also result in resistance to similar drugs that have never been used. Thus, knowledge of the cross-resistance patterns of various antiretroviral agents is important when changing therapy.
Resistance mutations are random events and the chance that a particular mutation will appear increases with higher levels of viral replication. The risk of development of resistance is directly related to a patient's compliance in taking the prescribed dosage at the appropriate time. Taking antiretrovirals at a reduced dosage results in drug levels at which selection for drug-resistant variants can occur. Likewise, taking only one or two antiretrovirals increases the chance that the virus may become resistant to one or both. Resistance testing is most useful in two situations: - Patients with newly diagnosed HIV infection to detect transmission of resistant virus - Patients on HAART who are failing therapy (i.e., viral load is detectable despite treatment) to guide decisions regarding therapy changes. However, it is important to keep in mind that resistance testing only gives reliable information about drugs a patient is currently taking; a patient may have virus with resistance mutations to certain drugs (particularly if they have been exposed to them previously), which may not be detected in the absence of the selective pressure of taking that drug. Genotypic resistance testing is now easily available through many major laboratories. The test is costly (~U.S. $300 to $500), but this expense is negligible when compared with the cost of continuing ineffective antiretroviral medications. The viral load usually has to be above 1,000 copies/mL for the test to be performed, and the results are generally reported as a series of mutations identified by a letter-number-letter sequence (e.g., M184V). The number is the codon where the mutation has been detected, the first letter is the wild-type amino acid at that codon, and the last letter is the amino acid that has been substituted for it (i.e., M184V is a replacement of methionine with valine at the 184 codon). Interpretation of genotypic resistance testing is complex because some isolated mutations confer high-level resistance to a medication, whereas in other cases multiple mutations or specific combinations of mutations are needed for resistance to develop. Updated databases of resistance mutations are available through a number of Internet sites, including the one maintained by the International AIDS Society-USA (www.iasusa.org). Results of resistance testing must be considered in conjunction with knowledge of what antiretrovirals the patient has taken in the past. Phenotypic resistance testing may be useful in situations where patients have multiple genotypic resistance mutations. If noncompliance is contributing to resistance, a new HAART regimen should not be prescribed until obstacles to compliance are addressed and resolved |
|
|
A 23-year-old female is found to be HIV infected after routine testing early in pregnancy. Her CD4 count at the time of diagnosis is 188 cells/mm3, her hematocrit is mildly low at 31% with an MCV of 89fl; the remainder of her blood tests are unremarkable. She is prescribed zidovudine and lamivudine (in the form of Combivir) with lopinavir/ritonavir (Kaletra); she is also prescribed TMP/SMX for pneumocystis prophylaxis. Three months later, on routine follow-up, she remarks that she has been feeling short of breath with exertion. Her hematocrit is now 22% with an MCV of 109fl.
Which ONE of her medications is the most likely cause of her anemia? a). Zidovudine b). Lamivudine c). Lopinavir/ritonavir d). TMP-SMX |
Answer - A; Zidovudine is associated with a macrocytic anemia.
|
|
|
Common board tested neurologic adverse effects from antiretroviral therapy?
|
Most patients who take efavirenz (brand name Sustiva and a component of Atripla) experience some CNS side effects, including drowsiness, dizziness, abnormal dreams, impaired concentration and exacerbation of psychiatric disorders. These are usually mild and subside in the first 2-4 weeks, but may be severe or persistent and require discontinuation of the medication. Taking efavirenz at bedtime may help minimize these side effects.
Tipranavir (Aptivus) has been associated with an increased risk of bleeding and intracranial hemorrhage. Stavudine (Zerit) and didanosine (Videx) have been associated with peripheral neuropathy. |
|
|
Cardiac Adverse effects and antiretroviral therapy?
|
A number of studies have found an association between antiretroviral therapy and the risk of myocardial infarction. The drugs implicated include the protease inhibitors as well as the NRTIs abacavir (Ziagen) and didanosine (Videx). The data is primarily from observational studies and is not consistent over all studies. (21) The protease inhibitors are thought to increase the risk of myocardial infarction at least partly through their effects on lipids. (22) The increased risk observed with abacavir and didanosine cannot be explained by lipid changes. (23) The DHHS guidelines recommend managing other risk factors for cardiovascular disease and avoiding these agents in patients with or at high risk for cardiovascular disease.
|
|
|
GI Adverse Effects and Antiretroviral therapy
|
Gastrointestinal side effects: nausea, vomiting and diarrhea, are common side effects with many agents, including all of the PIs and the NRTIs, zidovudine, and didanosine. Patients should be advised that the symptoms may get better with time. Taking the medication with food may help with these side effects, although some medications are recommended on an empty stomach. Taking antiemetics or antidiarheals may help control the symptoms. Didanosine is also associated with pancreatitis.
|
|
|
Hepatobiliary side effects and antiretroviral therapy
|
Hepatotoxicity in the form of clinical hepatitis or asymptomatic elevations in liver enzymes has been reported with all of the NNRTIs, most NRTIs and maraviroc (Selzentry). The NNRTI nevirapine (Viramune) has been associated with severe hepatotoxicity and hepatic failure, which generally occurs during the first 6 weeks of therapy. There have also been reports of severe hepatoxicity associated with the PI tipranavir. (24) The NRTIs, particularly zidovudine, stavudine and didanosine are associated with a syndrome of lactic acidosis and hepatic steatosis, which may be accompanied by pancreatitis. This syndrome is due to mitochondrial toxicity and presents with a nonspecific gastrointestinal prodrome (nausea vomiting and abdominal pain) and may progress to jaundice, mental status changes, respiratory distress and multiorgan failure.
Two of the protease inhibitors, atazanavir (Reyataz) and indinavir (Crixivan) are associated with a clinically benign unconjugated hyperbilirubinemia, which may cause jaundice in some individuals with a genetic predisposition. |
|
|
Renal adverse effects and antiretroviral therapy
|
The NRTI tenofovir (brand name Viread, also a component of Truvada and Atripla) is associated with renal toxicity that may occur weeks to months after initiation of therapy; severe toxicity is rare. Tenofovir has also been associated with cases of Fanconi's syndrome, which typically presents with hypokalemia, hypophosphatemia and normal anion gap metabolic acidosis. Nephrolithiasis may occur with the PIs indinavir and to a lesser extent, atazanavir and fosamprenavir.
|
|
|
Hematologic adverse side effects and antiretroviral therapy
|
The NRTI zidovudine may cause bone marrow suppression with anemia (usually macrocytic) and neutropenia; severe anemia and neutropenia are rare.
The PI tipranavir is associated with an increased risk of bleeding and intracranial hemorrhage. |
|
|
Endocrine adverse side effects and antiretroviral therapy
|
All of the PIs are associated with hyperlipidemia, except for atazanavir when used alone (i.e. without ritonavir boosting). Antiretroviral medications are also associated with fat redistribution. Lipodystrophy, which presents with loss of fat in the face, extremities and buttocks, is associated with the NRTIs, particularly stavudine and to a lesser extent zidovudine and the others. Lipohypertrophy, which presents with increase abdominal girth, breast size and dorsocervical fat pad ("buffalo hump"), is seen with PI- or NNRTI-based regimens with stavudine or zidovudine.
|
|
|
Cutaneous adverse side effects and antiretroviral therapy
|
Rash is a side effect of the NNRTIs, particularly nevirapine. Rash usually occurs a few days or weeks after initiation of therapy and may progress to Stevens-Johnson syndrome and toxic epidermal necrosis. Less severe rash has also been reported with most of the PIs.
|
|
|
Hypersensitivity reactions and antiretroviral therapy
|
Abacavir (brand name Ziagen, also a component of Epzicom and Trizivir) is associated with a hypersensitivity reaction that usually occurs in the first week or two of taking the medication. Symptoms include fever, rash, headache, myalgias, diarrhea, vomiting, and abdominal pain, and may progress to hypotension, respiratory distress and death, especially if the medication is continued or the patient is rechallenged after an initial reaction. Most cases occur in individuals who are HLA B*5701 positive, and it is recommended that everyone be screened for this prior to prescribing abacavir (or any of the combination products that contain abacavir). Patients should not be rechallenged with this drug if a hypersensitivity reaction is suspected, even if they are HLA B*5701 negative.
|
|
|
Drug interactions to consider with antiretroviral therapy
|
The most important drug interactions are those with the PI ritonavir, which is a potent inhibitor of the cytochrome P450 enzyme CYP3A4. Ritonavir is used for this reason to boost other protease inhibitors, but this also leads to numerous potential interactions with other drugs that are metabolized by this pathway. These drugs include the antifungal ketoconazole, the antimyobacterials rifabutin and rifampin, the benzodiazepines midazolam and triazolam, the HMGcoA reductase inhibitors (i.e. statins) lovastatin and simvastatin, and all of the phosphodiesterase inhibitors (sildenafil, tadalafil, and vardenafil). Ritonavir can also reduce the metabolism of a number of corticosteroids; there have been reports of iatrogenic Cushing's syndrome associated with the concurrent use of inhaled/intranasal fluticasone, inhaled budesonide, and triamcinolone injections; most of the reports have been with fluticasone. The guidelines recommend that some of these drugs not be given with ritonavir (including rifampin, midazolam, triazolam, lovastatin and simvastatin); for other drugs, reduced starting dose and closer monitoring are generally recommended.
Another important drug interaction is between medicines that lower stomach acid and the PI atazanavir (Reyataz). It is recommended that proton pump inhibitors not be used with this drug, particularly if the atazanavir is not boosted with ritonavir. H2 blockers may be given, but it is recommended that the atazanavir be given at least 2 hours before and 10 hours afterwards; for antacids, it is recommended that atazanvir be given at least 2 hours before or 1 hours after. |
|
|
A 25 year-old female presents with an acute febrile illness with cough, sore throat, sinus congestion and runny nose. She is a commercial sex worker, but says that her partners consistently use condoms. She had a serum HIV test (EIA) three months ago, which was negative. A rapid HIV antibody test is negative, and HIV viral load is 800.
Which one of the following is the most likely explanation for her test results? a). She has acute HIV infection. b). She has chronic HIV infection and a false negative rapid HIV antibody test. c). She is not HIV-infected and her viral load is a false positive test. d). She has been exposed to HIV and is clearing the infection. |
Answer C - she is not HIV infected and her viral load is a false positive test
The most likely explanation is a false positive HIV RNA; the false positive rate of the HIV RNA test is about 3% and these are generally at low levels (<10,000 copies/ml). This viral load is not consistent with acute infection when viral load is very high (>100,000). Chronic HIV infection is unlikely since the rapid HIV test is highly sensitive and she had a recent serum test which was negative. While there are some individuals who do not become infected despite high levels of exposure, this would not be a likely explanation for these test results. |
|
|
A 32 year-old female was diagnosed with HIV infection two years ago. Her initial CD4 count was 40 and she was started on trimethoprim-sulfamethaxazole (TMP-SMX) daily and azithromycin weekly. She was also started on antiretroviral therapy and has had an increase in her CD4 count to 230 and 270 on her two most recent testing. Her viral loads have been consistently undetectable. Which one of the following would be the best option at this time?
a). Continue azithromycin and stop TMP-SMX. b). Stop azithromycin and TMP-SMX. c). Continue azithromycin and TMP-SMX. d). Stop azithromycin and gradually taper the dosage of the TMP-SMX. |
Answer B - Both can be stopped once the CD4 count has risen beyond the threshold for starting them (50 for azithromycin and 200 for TMP-SMX).
|
|
|
A 51 year-old female with HIV infection has been seeing you for several years. Her initial CD4 counts were above 500 and viral loads in the 1,000's without antiretroviral therapy. However, her two most recent CD4 counts were 450 and 470; her most recent viral load was 11,000. Which one of the following would the current guidelines recommend?
a). Hold off antiretroviral therapy until the CD4 count is below 350. b). Discuss initiating antiretroviral therapy at this time. c). Hold off antiretroviral therapy until the HIV viral load is over 100,000. d). Strongly recommend initiating antiretroviral therapy at this time. |
Answer B - discuss initiating antiretroviral therapy now
The current guidelines recommend considering therapy once the CD4 count has fallen below 500. This should be discussed with the patient and she should be given the option to begin therapy now. The guidelines make recommendations based on CD4 count; viral load is not a consideration. |
|
|
A 41 year-old male with HIV infection is seen for routine follow-up. He has never been on antiretroviral therapy and his most recent CD4 count was 525 and viral load 23,000. He wants to start antiretroviral therapy because he has heard of recent studies that suggest that earlier therapy is beneficial. He also wants to reduce the risk of transmitting HIV to his uninfected partner. Which one of the following options would be the best choice for this patient?
a). Do not prescribe antiretroviral therapy until CD4 count is below 500. b). Prescribe zidovudine for now and add other drugs if his CD4 count falls below 500. c). Prescribe tenofovir, emtricitabine and efavirenz in the single-pill formulation (Atripla). d). Prescribe tenofovir and emtricitabine for now and add a third drug if the viral load is still detectable in three months. |
Answer C
This patient should be given the option of starting antiretroviral therapy at this point and this is a reasonable choice for initial therapy. If the decision is made to begin antiretroviral therapy, the patient should be prescribed a minimum of three agents. Prescribing one or two agents carries a high risk of resistance. |
|
|
A 26 year old graduate student presents for HIV care. He describes himself as having a "nervous stomach", takes omeprazole and Tums frequently, and is very worried about the GI intolerance of antiretroviral therapy. He has a K103N mutation that confers resistance to efavirenz detected on his baseline genotype.
What would be the best starting regimen in this patient? a). Once daily coformulation of tenofovir, emtricitabine and efavirenz b). Once daily regimen of tenofovir, emtricitabine and atazanavir boosted with ritonavir c). Once daily regimen of tenofovir, emtricitabine and darunavir boosted with ritonavir d). Twice daily regimen of tenofovir, emtricitabine and raltegravir |
Answer D - Twice daily regimen of tenofovir, emtricitabine and raltegravir
The regimen of tenofovir, emtricitabine and raltegravir is the most likely to be effective and have the least side effects for this patient. The first regimen is not a good choice because the virus is resistant to efavirenz. The second is not a good choice because atazanavir should not be given with antacids. The third option is likely to have gastrointestinal side effects. |
|
|
A 47 year-old female has been on antiretroviral therapy for the past seven years, most recently with tenofovir, emtricitabine, and efavirenz. She has done well in the past and has always had undetectable viral loads. She went on a one-week cruise two months ago and forgot to take her medication with her. She has been taking it consistently since then. Her CD4 count is 627, viral load 10,200. Genotypic resistance testing shows a K103N mutation, which confers resistance to efavirenz. Which one of the following options would be best for the patient?
a). Add ritonavir to boost the current medications. b). Continue current regimen and change if the CD4 count falls below 500 or the viral load increases to above 100,000. c). Stop all antiretroviral medications and start with a new regimen when the CD4 count falls below 350. d). Stop efavirenz and start atazanavir and ritonavir. |
Answer D - Stop Efavirenz and start atazanavir and ritonavir
The HIV virus has become resistant to efavirenz, so the best option would be to replace it with another effective drug, in this case ritonavir-boosted atazanavir. Ritonavir is used to boost other protease inhibitors (PIs); the patient is currently not taking any PIs and ritonavir would not be effective alone. Continuing a regimen that is not suppressing viral replication will lead to further resistance mutations, rendering other drugs that she is taking ineffective. Stopping an effective regimen and restarting when the CD4 count falls has been shown to be associated with an increased morbidity and mortality. |
|
|
A 54 year-old male with HIV infection diagnosed 10 years ago comes for routine follow-up. He has been on antiretroviral therapy for the past 7 years and has done well. He is currently taking zidovudine, lamivudine and nevirapine and denies missing any doses in the past 3 months. Three months ago, his CD4 count was 860 and his viral load <50 copies/ml. At this visit, his CD4 count is 716 and viral load 110 copies/ml. Which of the following should be done at this time?
a). Continue current medications; no other testing necessary. b). Check a genotype for resistance mutations. c). Change his regimen to tenofovir, emtricitabine and efavirenz. d). Add raltegravir to his regimen. |
Answer A - continue current meds
A transiently mildly detectable viral load can occur and is generally not a sign of resistance. The decreased CD4 count is likewise not a concern, since CD4 counts can vary. If resistance is a concern, resistance testing should be done before making changes; however, the viral load is not high enough to do resistance testing. |
|
|
A 36 year old female with recently diagnosed HIV infection is prescribed Epzicom (abacavir + lamivudine) and atazanavir (Reyataz) boosted with ritonavir. Two weeks after starting the medication, she calls you in the evening and says she has developed a rash, nausea, vomiting and diarrhea.
Which ONE of the following would be the best course of action? a). Continue current regimen and treat symptomatically. b). Stop the ritonavir and continue all other drugs. c). Stop all of the antiretroviral medications immediately. d). Have her come in to have an HLA B*5701 checked. |
Answer C - stop all of the ART stat
This patient's symptoms are consistent with a hypersensitivity reaction to abacavir; she should stop the medication and be evaluated urgently. Screening for HLA B*5701 prior to prescribing abacavir greatly reduces the risk of this reaction, but the drug should be stopped on anyone who is suspected of having a reaction and they should not be rechallenged. |
|
|
What are the common symptoms associated with the acute phase of HIV?
|
The acute phase of HIV infection is associated with nonspecific symptoms common to many viral infections, including fever, sore throat, malaise, swollen lymph nodes, and ofter a transient maculopapular rash.
The symptoms are probably a result not only of virus replication but also the immune response that is being generated. |
|
|
Where does replication of HIV occur?
|
In the early days after infection, virus replication increases dramatically, with plasma viral loads reaching an excess of 10 million virus particles per milliliter of plasma in most persons.
This replication occurs mainly in CD4-bearing lymphocytes in gut-associated lymphoid tissue (GALT) and particularly in those bearing the CCR5 co-receptor required for virus entry. This early and massive infection results in a profound reduction in CD4 cell number in GALT, accompanied by a much more modest decline in the peripheral blood CD4 cell count. Estimates are that more than 50% of memory CD4 cells are infected and lost in this early phase of infection. |
|
|
How long does it typically take before reaching a steady-state viremia level in HIV?
|
The initial peak in viremia is brought to an average steady-state level of about 30,000 copies within 6 months of infection, most likely as a result of the generation of partially effective antiviral immune responses.
Among these responses is the generation of virus-specific CD4+ helper T cells, which orchestrate a coordinated humoral and cellular immune response. Because CD4 is a receptor for the virus and HIV has the unique ability to infect activated CD4 cells preferentially, these cells that are attempting to respond to the virus are infected and probably partially deleted in the early phase of infection when the viral load is so high. This leaves most infected persons with an impaired ability to fight HIV infection. Lymphocytes bearing the CD8 cell surface marker, called cytotoxic T lymphocytes, are generated and their appearance correlates with the initial dramatic decrease in plasma viral load. Functioning of these cells is impaired in the absence of an adequate virus-specific CD4+ helper T-cell response, however, thus explaining the lack of effective long-term control of infection. The difficulty with immune control is exacerbated by the continued evolution of the virus within an infected person. Infidelity of the viral reverse transcriptase allows the generation of multiple closely related, yet distinct viruses within a given person. In the same way that partial antiviral drug pressure allows the generation of drug resistance mutations, partial antiviral immune pressure permits the gradual development of mutations within key sites targeted by the immune responses that have been generated. |
|
|
What is the underlying reason why an HIV vaccine is so difficult to develop?
|
The antibody response to HIV is very slow and cumbersome to develop.
Antibodies can generally be detected within a few weeks of infection; many of these antibodies are directed against virion debris, and a smaller number can neutralize infectious virus. However, as these antibodies are generated, viral mutations in the envelope protein ensue and allow the virus to escape immune detection. New waves of neutralizing antibodies are generated, but the virus continues to outpace this response by the development of additional mutations in the highly variable envelope protein. The ability of the virus to escape neutralizing antibodies is one of the major problems with HIV infection and represents a major hurdle for vaccine development. Those antibodies that do develop are usually only weakly neutralizing, in part because of the heavy sugar coating on the envelope of the virus that keeps crucial neutralization sites masked from the immune system. |
|
|
Typically, how often do you have to have your CD4 count and Viral load checked?
|
Every 3-4 months
|
|
|
How do you define AIDS?
|
Patients with CD4 counts <200 or a CD4 % <14% meet CDC criteria for AIDS, even in the absence of an HIV-related opportunistic infection.
|
|
|
What is "Advanced HIV Disease"?
|
CD4 <50 --> patients are at particularly high risk for opportunistic infections and HIV-related death.
|
|
|
What is the most important predictor of clinical outcome when starting ART?
|
Recovery of the CD4 count in response to ART has been shown to be the most important predictor of clinical outcome, even more than the virologic response.
|
|
|
At what CD4 count is pneumococcal safe to give?
|
>200
|
|
|
What factors are associated with poor CD4 count response after ART initiation?
|
Factors associated with poor CD4 responses:
- older age - higher baseline HIV RNA levels - low CD4 cell counts - Hepatitis C coinfection - Receipt of a non-protease inhibitor-containing regimen. - Genetic factors (patients CCR5 and multidrug resistance (MDR1) transporter genotype |
|
|
What is the HIV RNA-CD4 "disconnect" phenomenon?
|
A different form of discordant HIV RNA and CD4 response is seen when there is a continued increase or stability in the CD4 cell count despite a rebounding HIV RNA level. This situation has been observed most commonly in patients treated with a regimen that includes a protease inhibitor and two nucleoside reverse transcriptase inhibitors and accounts in part for the ongoing excellent prognosis of HIV patients receiving this therapy despite the high rates of virologic breakthrough seen in clinical practice. Several possible explanations for this HIV RNA–CD4 “disconnect” phenomenon have been proposed. They are not mutually exclusive and include (1) emergence of antiviral-resistant HIV strains with impaired virulence (“fitness”) as compared with wild-type virus, (2) reduced CD4 cell turnover as compared with untreated controls, and (3) the prolonged time that the HIV RNA level remains below the pretreatment baseline, even after clear emergence of drug resistance. The duration of this immunologic benefit from ART despite virologic rebound varies among patients, but in one study it persisted for a mean of 72 weeks. In another study, the rate of CD4 decline was substantially slower in patients treated with ART who had multidrug-resistant virus than in those with wild-type HIV not receiving treatment, even with the same viral load. In this study, a viral load lower than 10,000 copies per milliliter or a reduction of viremia to 2 log below the pretreatment set point was associated with a CD4 gain, regardless of whether the viral load was undetectable.
|
|
|
What is Immune Reconstitution Inflammatory Syndrome?
|
This occurs when sudden improvement in immune function after starting ART can sometimes lead to dramatic worsening of preexisting opportunistic infections.
It probably results from eliciting a previously weak or absent host inflammatory response that becomes much stronger as a result of ART. |
|
|
What lung cancer is associated most with HIV?
|
Adenocarcinoma -- most common non-AIDS defining cancer in HIV patients.
|
|
|
Bilateral diffuse ground glass opacities
|
Think - PJP
No reticulonodular, not interstitial, not a consolidation! |
|
|
Spontaneous pneumothorax in a patient with HIV is...
|
...PJP related until proven otherwise!
PJP can lead to cysts in the lung! |
|
|
The fining that best predicts that a symptomatic patient does NOT have PJP is...
A) normal CXR B) induced sputum with no organisms C) CD4 cell count 400 D) PaO2 = 85 |
C - CD4>300
|
|
|
Following an episode of PJP, a patient is started on ART and TMP-SMX 1 DS daily. 6 mo later, he feels well; the CD4 increased from 55 to 300. All this continues for another 3 months.
What do you now recommend? |
Stop PJP prophylaxis but don't stop ARTs
|
|
|
Cause of mediastinal lymphadenopathy in an HIV patient is...
|
TB...until proven otherwise!
|
|
|
CMV pneumonia in patients with AIDS is...
a) usually found in drug users b) dx by serologic studies c) dx by culture of BAL fluid d) dx by histopath or cytology |
d - histopath and cytology diagnoses --> look for owl eyes!
CMV is rare in HIV but is found in BMT patients - different type of immunocompromised condition; usually found in gay men not drug users. BAL, sero don't help! |
|
|
Invasive Pulmonary Aspergillosis in HIV
|
Cases were more likely than controls to have:
- Neutrophils counts <1000 (neutropenic) - CD4 <30 - Used corticosteroids - Prior PCP - Death during study Wallace JM et al CHEST 1998; 114: 131-137 |
|
|
Most common neoplastic disorders in HIV
|
Lung Adenocarcinoma - 2x increased incidence irrelevant of smoking
Kaposi's sarcoma of the lung Lymphoma - usually B cell assoc with HHV8 --> poor px |
|
|
All of the following are characteristic radiographic findings in thoracic Kaposi's sarcoma, EXCEPT:
a) Pleural Effusion b) Mediastinal lymphadenopathy c) Kerley-B lines d) Pneumothoraces |
d- Pneumothorax
KB lines -- lymphangitic filling of tumor along bronchovascular bundles that cause appearance of fluid overload --> kerley b lines Bronchoscopy -- may show black-purple spots; don't have to biopsy |
|
|
Pulmonary HTN in HIV
|
WHO I
1/200 cases of HIV infection Not related to HIV infection of pulmonary vascular endothelium Unrelated to CD4 Treat like IPAH Prognosis is generally poor |
|
|
45 yo IDU is admitted with fever, weight loss, and RLL opacity.
Sputum AFB smear is positive --> RIPE started HIV+ --> ART started He improves --> d/c on DOT but returns 2 weeks later with fever, otherwise well. CXR shows NEW mediastinal lymphadenopathy and R pleural effusion a) Bronchoscopy with BAL and TBNA of mediastinal lymphadenopathy b) streptomycine and ciprofloxacin c) STOP ART d) Observation |
d) Observation -- patient has IRIS
Can give conservative supportive tx, NSAIDs |
|
|
Sarcoidosis-like pulmonary disorder after ART
|
Related to CD4/CD8 count -- Naccache JM, et al AJRCCM 1999; 159:2009-2013
|

