![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
What is an ion? |
An atom or molecule with a net electric charge due to the loss or gain of one or more electrons |
|
|
What is an acid, base and buffer ? |
Acid - a chemical substance that neutralizes alkalis dissolves some metals, and turns litmus red: typically , a corrosive or sour-tasting liquid of this kind. Base- a substance capable of reacting with an acid to form a salt and water , or ( more broadly) of accepting or neutralizing hydrogen ions. Buffer- a solution that resists changes in pH when acid or alkali I'd added to it. Buffers typically involve a week acid or alkali together with one of its salts. |
|
|
What's the pH balance of blood? |
It's typically regulated between 7.35 and 7.45 |
|
|
What are neutral, acidic, and alkaline pH levels? |
Neutral -pH of 7 Acidic- less than 7 Alkaline - greater than 7 |
|
|
What's a molecule? |
A group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction. |
|
|
How is an ion formed? |
To form an ion- you need to lose or gain electrons in order to fulfill outer valence electron shells. |
|
|
What "holds" atoms together in a molecule? |
Molecules are held together by electrons shared between bonded atoms. |
|
|
Synthesis Reaction |
The joining together of two reactants (compounds) to produce a complex product compound. D + R ------> DR |
|
|
Decomposition Reaction |
Is a type of chemical reaction in which a single compound breaks down into a two of more elements or new compound. AZ--> A+ Z. |
|
|
Double Replacement Reaction |
A type of chemical reaction where two compounds react, and the positive ions ( cation) and the negative ions (anion) of two reactants switching places, forming two new compounds or products. AB + CD ---> CB + AD |
|
|
Oxidation-reduction Reaction |
A type of chemical reaction that involves a transfer of electrons between two species. |
|
|
What's an Atom ? |
The smallest component of an element having the chemical properties of the elements, consisting of a nucleus containing combinations of neutrons and protons and one or more electrons. |
|
|
What's a Proton ? |
A stable subatomic particle occurring in all atomic nuclei, with a positive electric charge equal in magnitude to that of an electron, but of opposite sign. |
|
|
What's a Neutron? |
A subatomic particle of about the same mass as a Proton but without an electron but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen. |
|
|
What's a Electron? |
A subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids. |
|
|
What's a chemical bond? |
Any of several forces, especially the ionic bond, covalent bond, and metallic bond, by which atoms or ions are bound in a molecule or crystal |
|
|
What's a Covalent Bond? |
A chemical bond that involves the sharing of electrons pairs between atoms. |
|
|
What's an Ionic Bond? |
The the electrostatic force of attraction between two opposite charged ions. |
|
|
What's replication ? |
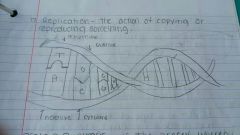
The action of copying or reproducing something. |
|
|
What's Transcription? |
The process by which genetic information represented by a sequence of DNA nucleotides is copied into a newly synthesized molecule of RNA, with the DNA serving as a template. |
|
|
Whats Protein Synthesis ? |
It's the process whereby biological cell generate new proteins; it's balanced by the loss of cellular proteins via degradation. |

