![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
|
What quantity of hemoglobin is synthesized each day? Why?
|
6-7g / day to replace heme lost through the normal turnover of RBCs
|
|
|
Where is most iron in the human body? How much?
|
~70% of total iron is present as heme iron in RBCs
|
|
|
What concerns does the body have to deal with as it breaks down heme?
|
- Handling the hydrophobic products of the porphyrin ring cleavage
- Retention, safe mobilization, and re-utilization of iron |
|
|
What form of iron can generate reactive oxygen species (ROS)?
|
Ferrous iron (Fe2+) can generate ROS by the Fenton reaction → promotes oxidative stress
|
|
|
What structure can free heme and heme iron negatively affect?
|
Lipid membranes
|
|
|
How does free heme and heme iron negatively affect lipid membranes?
|
- Heme is hydrophobic and can intercalate into lipid bilayers
- Heme iron can generate ROS by Fenton Reaction - ROS participate in oxidation of lipid membrane components and cause lipid peroxidation |
|
|
What effect do structural changes to lipid membranes caused by ROS produced by heme iron have?
|
- Alters fluidity and channels
- Alters membrane-bound signaling proteins - Increases ion permeability |
|
|
What is the effect of lipid peroxidation caused by ROS produced by heme iron?
|
Lipid peroxidation products form adducts and cross-links with non-lipids (eg, proteins and DNA)
|
|
|
What ROS does ferrous iron (Fe2+) help form?
|
1) Fe2+ + O2 → Fe3+ + O2˙-
2) 2 O2˙- + 2H+ → H2O2 + O2 3) H2O2 + Fe2+ → Fe3+ + HO˙ + OH- O2˙- = Superoxide radical HO˙ = Hydroxyl radical |
|
|
What type of endothelium is affected by free plasma hemoglobin and heme?
|
Toxic to vascular endothelium
|
|
|
What effect does HEME IRON have on vascular endothelium?
|
- Heme iron promotes oxidative stress
- Can cause endothelial activation responsible for vaso-occlusive events and thrombus formation |
|
|
What effect does FREE HEMOGLOBIN have on vascular endothelium?
|
- Scavenges NO (NO binds to heme iron)
- Reduces NO bioavailability - This prevents smooth muscle relaxation (usually achieved via GC→ cGMP) - Dysregulation of endothelium vasodilator-vasoconstrictor balance can lead to severe VASOCONSTRICTION and HTN, a common feature of pathologic conditions associated with hemolysis |
|
|
What pathologic conditions are commonly associated with hemolysis? Why?
|
- Severe vasoconstriction and HTN
- Caused by free hemoglobin scavenging NO (which leads to dysregulation of the endothelial vasodilator - vasoconstrictor balance) |
|
|
Which of the following statements is incorrect?
a) Heme can intercalate into a lipid bilayer b) Heme iron can generate ROS c) Hemoglobin cannot bind NO d) Lipid peroxidation can alter fluidity of membranes e) NO triggers vasodilation |
Hemoglobin cannot bind NO is incorrect
|
|
|
What happens to old RBCs?
|
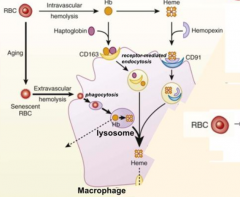
- RBCs become smaller in size and cell surface is modified with aging
- They become senescent → Extravascular Hemolysis → phagocytosed by macrophage |
|
|
What are the two ways that RBCs can be lysed? Which type predominates?
|
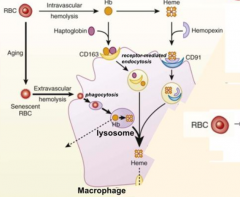
- Extravascular hemolysis (80-90%)
- Intravascular hemolysis (10-20%) |
|
|
What happens to RBCs undergoing Intravascular Hemolysis?
|
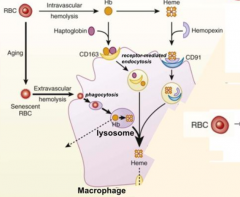
1) RBC membrane is perturbed releasing Hb and sometimes heme from Hb
2) Haptoglobin binds free Hb and Hemopexin binds free heme 3) Haptoglobin-Hb and Hemopexin-Heme can each be taken up by scavenger receptors via Receptor-Mediated Endocytosis into macrophages (CD163 for Hb and CD91 for Heme) 4) Heme is degraded to produce bilirubin, which is excreted into bile by liver |
|
|
Which molecule binds free Hemoglobin? How is this complex taken up by receptor mediated endocytosis?
|
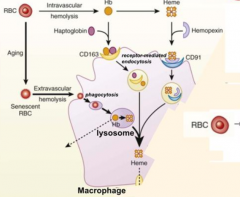
- Haptoglobin
- CD163 |
|
|
Which molecule binds free Heme? How is this complex taken up by receptor mediated endocytosis?
|
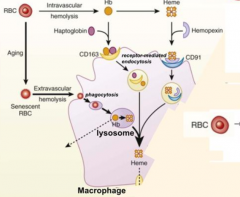
- Hemopexin
- CD91 |
|
|
What happens if there are reduced Haptoglobin levels when there is acute intravascular hemolysis?
|
- Hb remains unbound
- Leads to hemoglobinuria - Renal tubules reabsorb → Hemosiderinuria - Can also excrete excess Hb → Possible iron deficiency |
|
|
Where does Extravascular Hemolysis take place?
|
Within macrophages of spleen and liver ("tissue macrophages")
- Red pulp macrophages of spleen - Kupffer cells of liver |
|
|
What does Intravascular Hemolysis require?
|
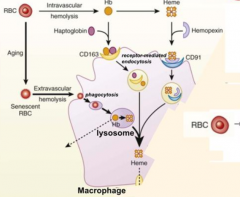
CD163 positive macrophages to scavenge hemoglobin-haptoglobin complex
|
|
|
How can you get an idea of how much Intravascular Hemolysis is happening?
|
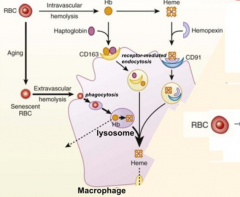
Measure Haptoglobin levels (the less haptoglobin there is the more intravascular hemolysis is happening)
|
|
|
What is Haptoglobin? What does it bind? Where is it made?
|
- Acute phase glycoprotein that binds free Hemoglobin (Hb)
- Nearly irreversible binding to Hb - Produced mostly in liver by hepatocytes and secreted into blood circulation |
|
|
When is there an increase in Haptoglobin levels?
|
During acute phase of inflammation and in neoplastic disease in response to inflammatory cytokines
|
|
|
What is Haptoglobin a marker of?
|
- Useful marker for inflammation-related diseases (levels increase)
- Clinical marker for hemolysis (levels decrease) |
|
|
What is the function of Haptoglobin?
|
Antioxidant:
- Following its release into plasma, Hb dissociates from tetramer into αβ dimers - Oxy-Hb dimers bind Haptoglobin tightly - Haptoglobin sequesters the dimers, preventing release of free heme and oxidative damage of heme iron Mechanism to conserve iron: - Haptoglobin-Hb complex can't pass through glomerular filter |
|
|
How are Haptoglobin-Hb complexes cleared from circulation if they cannot pass through the glomerular filter?
|
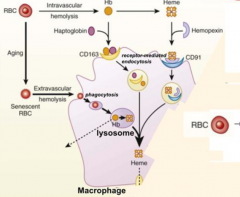
Hb scavenger receptor CD163 that is expressed on circulating monocytes and tissue macrophages
|
|
|
What are the structures of the phenotypes of Haptoglobin?
|
3 phenotypes of Haptoglobin:
- Hp1-1 - Hp2-1 - Hp2-2 Each contains 2 α-chains and 2 β-chains - All have the same β-chains - Two types of α-chains (1-83aa and 2-142aa) which is denoted in their names |
|
|
Which phenotype of Haptoglobin is found to be over-represented in auto-immune and inflammatory diseases?
|
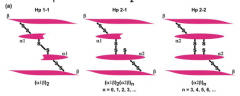
Hp2-2
|
|
|
How do the different phenotypes of Haptoglobin affect HDL?
|
Hp1-1:
- Preserves HDL function Hp2-2: - Defective HDL function |
|
|
How do the different phenotypes of Haptoglobin differ in size and antioxidant capacity?
|
Hp1-1:
- Small dimer - Highest antioxidant capacity Hp2-2: - Large cyclic polymer - Lowest antioxidant capacity |
|
|
What type of Haptoglobin preserves HDL function? Implications?
|
Hp1-1 genotype:
- ↑ reverse cholesterol transport - ↑ antioxidant activity |
|
|
What type of Haptoglobin causes defective HDL function? Implications?
|
Hp2-2 genotype:
- ↓ reverse cholesterol transport - ↓ antioxidant activity |
|
|
What is the mechanism of Receptor-Mediated Endocytosis for Haptoglobin-Hb?
|
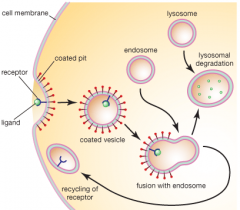
1) Haptoglobin-Hb complex binds CD163 receptor on macrophages
2) Membrane is coated with clathrin 3) Clathrin-coated vesicle pinches off and clathrin dissociates to return to membrane 4) Vesicle fuses with early endosome which acidifies via V-type ATPase 5) CD163 receptor releases Haptoglobin-Hb complex and is recycled back to cell membrane 6) Haptoglobin-Hb complex is digested in lysosome |
|
|
What is CD163? What does it bind? Where is it expressed?
|
- Type I Transmembrane Glycoprotein that contains 9 scavenger receptor cysteine rich (SRCR) domains
- Binds to Haptoglobin-Hb complex - Expression restricted to monocyte / macrophage lineage; recycles between early endosomes and plasma membrane |
|
|
Which of the following is not required for CD163 scavenger receptor expression on the cell surface?
a) signal recognition particle (SRP) b) golgi c) endoplasmic reticulum d) ubiquitination e) signal sequence |
Ubiquitination
|
|
|
What happens when Haptoglobin's buffering capacity is overwhelmed?
|
Hb undergoes a rapid conversion to metHb, liberating heme
|
|
|
What happens to the heme that is liberated when the haptoglobin buffering system for Hb is overwhelmed?
|
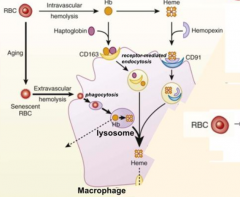
Ferriheme binds to albumin and other plasma components including lipoproteins and is subsequently transferred to Hemopexin
|
|
|
What is Hemopexin? What does it bind? Where is it made?
|
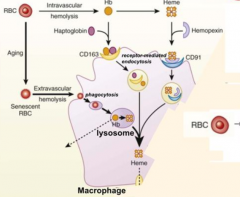
- Acute phase glycoprotein that binds free heme
- High binding affinity for heme (higher than albumin) and coordinates heme via its conserved clustering of histidine residues - Produced mostly in liver by hepatocytes and secreted into blood circulation |
|
|
When are Hemopexin levels elevated?
|
- During acute phase of inflammation in response to inflammatory cytokines
- During heme overload |
|
|
What is CD91? What does it bind? Where is it expressed?
|
- Type I Transmembrane Glycoprotein that is also known as the LDL receptor-related protein (LRP1)
- Binds to Hemopexin-Heme complex - Expression is found in numerous cell types (NOT restricted to macrophages); recycles between early endosomes and plasma membrane |
|
|
What happens to hemoglobin components in the lysosome?
|
- Globin proteins are degraded to amino acids in lysosome
- Heme is transferred to cytosol where it is catabolized by Heme Oxygenase-1 (HO-1) that is associated w/ ER membrane w/ activate site facing cytoplasm |
|
|
What does heme degradation produce?
|
Bilirubin which is normally excreted into bile by liver
|
|
|
What can result if there are elevated levels of bilirubin int he skin and sclera?
|
Jaundice or icterus - imparts a yellow color to these tissues
|
|
|
What can cause hyperbilirubinemia?
|
Inherited disorders of abnormal bilirubin metabolism
|
|
|
Where does heme catabolism occur?
|
~80% occurs in senescent erythrocytes
~20% comes from turnover of immature RBCs and various cytochromes in non-erythroid tissues |
|
|
What happens to senescent RBCs?
|
They are taken up by macrophages (extravascular hemolysis) of the reticuloendothelial system of the liver and spleen
|
|
|
What is bilirubin? What color?
|
- Orange pigment derived from degradation of heme proteins
- Potentially toxic waste product that is generally harmless because of binding to serum albumin |
|
|
Why is bilirubin generally harmless despite having toxic potential?
|
It binds to serum albumin
|
|
|
How does the heme ring open mechanistically?
|
1) Ferroprotoporphyrin IX ring is selectively cleaved at α-methene bridge
2) Non-enzymatic oxidation by molecular O2 w/ elimination of CO 3) Iron is released after addition of e-, releases green pigment biliverdin 4) Conversion of biliverdin (green) to bilirubin (orange) in macrophage 5) Binding of bilirubin to albumin 6) Uptake, storage, conjugation, and excretion of bilirubin in liver |
|
|
What happens during the first step of heme catabolism?
|
- Ferroprotoporphyrin IX ring is selectively cleaved at α-methene bridge
- Heme Oxygenase (HO-1) catalyzes reaction and requires e- from NADPH cytochrome P450 Oxidoreductase (CYPOR) |
|
|
What happens during the second step of heme catabolism, after opening of α-methene bridge in Ferroprotoporphyrin IX ring?
|
- Non-enzymatic oxidation by molecular O2 w/ elimination of CO (CO is bound by hemoglobin)
- Only known reaction in human tissues and cells that produces CO as byproduct |
|
|
What happens during the third step of heme catabolism, after the non-enzymatic oxidation by molecular O2, which eliminates CO?
|
- Iron is released after addition of e- (Fe2+/3+ is bound by Ferritin)
- Releases green pigment biliverdin |
|
|
What happens during the fourth step of heme catabolism, after Biliverdin is released?
|
- Conversion of biliverdin (green) to bilirubin (orange) in macrophage
- Catalyzed by Biliverdin Reductase, which uses either NADH or NADPH for activity - Bilirubin is less polar than biliverdin and crosses membranes more readily - Bilirubin is an anti-oxidant and appears to be particularly important during neonatal period, when concentrations of other antioxidants are low in body fluids |
|
|
What happens during the fifth step of heme catabolism, after Biliverdin is reduced to Bilirubin?
|
- Binding of bilirubin to albumin (in blood) tightly but reversibly
- Albumin keeps bilirubin in solution and transports it from primary sites of production (spleen and BM) to primary site of excretion (liver) - Uptake, storage, conjugation, and excretion of bilirubin in liver |
|
|
What is the significance of the changing of colors in heme catabolism?
|
Biliverdin (green) converted to Bilirubin (orange)
|
|
|
Which of the following is NOT a component that serves a protective function during intravascular hemolysis?
a) antibody b) haptoglobin c) hemopexin d) CD91 e) CD163 |
Antibody (part of extravascular hemolysis)
|
|
|
Which enzyme converts heme to a linear molecule?
|
Heme Oxygenase (HO-1) which requires NADPH Cytochrome P450 Oxidoreductase (CYPOR)
|
|
|
What enzyme removes iron during heme catabolism?
|
Heme Oxygenase (HO-1) which requires NADPH Cytochrome P450 Oxidoreductase (CYPOR)
|
|
|
What enzyme produces carbon monoxide (CO)?
|
Heme Oxygenase (HO-1) which requires NADPH Cytochrome P450 Oxidoreductase (CYPOR)
|

