![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
LIMESTONE CYCLE |
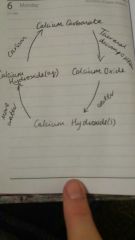
CaCO3 |
|
|
|
CEMENT |
Limestone + Clay Mixed in a kiln |
|
|
|
MORTAR |
Cement + Water + Sand Used to hold bricks together. |
|
|
|
CONCRETE |
Cement + Water + Sand + Aggregate. Used to make paths and roads. |
|
|
|
Pro's of LIMESTONE QUARRY |
•Adds more trade to the area. •Can be turned into a leasure area after. •Brings more Jobs. |
|
|
|
Con's of LIMESTONE QUARRY |
• Dust adds to pollution • CO2 from trucks adds to global warming •Destroys habitats. •Increases traffic. |
|
|
|
Qualities of COPPER |
• Hard • Good conductor • Not reactive to water. • Strong • Can be bent |
|
|
|
Qualities of ALUMINIUM |
• Corrosion-ressistant • Not a strong metal unless it's an alloy. • Low density metal |
|
|
|
Qualities of TITANIUM |
• Corrosion-resistant • Very strong • Very hard • Can be bent • Low density metal |
|
|
|
How to prevent erosion in metals |
Paint e.g on bridges |
|
|
|
EXTRACTED IRON |
Aka Impure iron. 4% impurities. Usually carbon. Cast Iron used for ornamental decorations. Brittle. |
|
|
|
PURE Iron |
Regular atom arrangement. Layers can slide over each other easily. Soft and you flexible. |
|
|
|
STEEL (Iron) |
Lots of metals and carbon mixed in. The layers can't slide over each other as easily. Hard and strong. |
|
|
|
Types of steel alloys (3) |
LOW CARBON STEEL >Easily shaped HIGH CARBON STEEL >Hard and inflexible STAINLESS STEEL >Corrosion-resistant. |
|
|
|
Reduction (extracting metals) |
Reduction by carbon where oxygen is removed. More reactive metals (than carbon) cannot be extracted through reduction. However less reactive metals can be. |
|
|
|
Electrolysis, why is it bad? |
More expensive because it uses lots of energy to make molten compounds. |
|
|
|
Displacement to extract metal |
Less reactive metals are "Kicked out" and replaced by the more reactive metals because they have stronger bonds. |
|
|
|
Electrolysis of Copper to make pure. |
•Impure copper at anode. •Electrons pulled off copper atoms at anode (+) making a solution of Cu2+ Ions. •These ions go towards the cathode (-) where they gain electrons and are made into copper atoms again. • Impurities dropped at anode. •Pure copper bonds to cathode |
|
|
|
Bioleaching method... |
Bacteria get energy from the bonds between the metal and impurities which separate the metal ore in the process. A product of this is LEACHATE solution that contains copper and can be filtered. |
|
|
|
Phytomining method... |
Growing plants in soil containing the impure metal. The plants cannot get rid of the metal or use it so it builds up. The leaves of the plants are then harvested and burned and the copper is extracted through the ashes. |
|
|
|
Why are Bioleaching and Phytomining bad economically? |
They are slow to extract metals and won't have as much of a profit. |
|
|
|
Pro's and con's of metal extraction. |
Pro's •Brings money to the area Brings more Jobs. Con's •Bad for the environment •CO2 released adds to global warming. •Habitats are destroyed •Abandoned mines are dangerous |
|
|
|
Why recycling metal is important. |
•Mining and extracting metals takes energy which is expensive and comes mainly from fossil fuels. •Fossil fuels are running out so we need to conserve them. •Burning fossil fuels contributes to acid rain, global warming and climate change. •There is a finite amount of metal in the earth so recycling conserves resources. •Throwing metal into landfills pollutes the surroundings |
|
|
|
How is crude oil formed and extracted? |
It is a fossil fuel formed by dead buried plants and animals. It is extracted through drilling. |
|
|
|
What is crude oil a mixture of? |
Hydrocarbons. Carbon and hydrogen that have no chemical bonds. |
|
|
|
What are the first 4 alkanes? |
Methane Ethane Propane Butane |
|
|
|
What is the process of fractional distillation? |
1. Crude oil is pumped in. 2. The vapourised oil rises. 3. Gas is constantly tapped off at the condensing levels. |
|
|
|
What is the formula for alkanes? |
CnH2n+2 |
|
|
|
Qualities of short carbon chains. |
•Less viscous (more runny) •More flammable •Lower boiling and condensing point |
|
|
|
Pro's and con's of crude oil |
Pro's •Easiest and cheapest thing to use for fuel. •More reliable
Con's •Non renewable •Oil spills kill animals •Birds covered in fuel are poisoned. •Burning crude oil adds to acid rain, global warming. |
|
|
|
Burning fossil fuels produces... |
Sulfure dioxide Carbon dioxide Carbon monoxide Nitrogen ( burned at high temps) Soot ( particulates) |
|
|
|
What does sulfuric acid do? |
Erode limestone and statues Makes lakes acidic which can kill plants and animals. |
|
|
|
How to reduce sulfur emissions. |
Reduce our usage of fossil fuels. Remove sulfur before. (More expensive and need a more energy) Power stations have acid has scrubbers to clean emissions.
|
|
|
|
What does CO2 cause? |
Global warming Global dimming ( reflects light ) Climate change e.g. flooding. |
|
|
|
Ethanol (Biofuel) pro's and con's |
Ethanol is made from fermenting plants so is carbon neutral. But engines need to be converted. Not widely available Increase food prices. |
|
|
|
Biofuel (not ethanol) pros & cons |
Biofuels are made from vegetable oil so it is carbon neutral. It can be mixed with diesel to make biodiesel so the engines don't have to be converted. It produces less sulfure dioxide however it is expensive and we cant make enough. The food prices would go up too. |
|
|
|
What has a double bond? |
An alkene |
|
|
|
What is the process of cracking? |
A thermal decomposition reaction. Hear a long chain hydrocarbon to vapourise it. The vapour passes over a powder catalyst (aluminium oxide) the long chain is split apart. |
|
|
|
How to test for an alkene. |
Add the substance to bromine water. If an alkene is present the water will turn from Orange to colourless. This is because the double bond has formed bonds with the bromine. |
|
|
|
How to make ethanol. |
Hydrate ethene over a catalyst with h2o. Ferment sugar and convert to ethanol us in yeast. |
|
|
|
How to make polymers from alkanes. |
Polymerisation. Small alkene monomers form long chain molecules called polymers. E.g. ethene = poly(ethene) polythene Propane = poly (propene) |
|
|
|
How temp affects polyethene |
200°C = flexible, low density 60°C = rigid, dense |
|
|
|
Uses for polymers |
Plastic bags. Lycra fibre tights Waterproof material Dental tooth fillings Hydrogel sound dressings. Biodegradable packaging Memory foam |
|
|
|
How to extract plant oils. |
Plant material is crushed. The oil is squashed out by metal. Lol is spun out by a centrifuge. (A lettuce spinner) Solvents can be used. Distillation refines oils, removes water, solvents and impurities. |
|
|
|
Emulsions are made from what? |
Oil and water. |
|
|
|
Unsaturated fats can either be: |
Monounsaturated C=C Polyunsaturated C=C C=C C=C (lots) |
|
|
|
Unsaturated oils do what in bromine water? |
Double bonds C=C
Discolour bromine water
Liquid at room temp but hardend when reacted with hydrogen. (Hydrogenation) |
|
|
|
Which is stronger, polythene or polyamide? |
Polyamide. |
|
|
|
Emulsifiers |
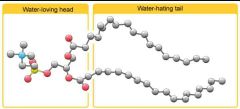
HYDROPHILIC HYDROPHOBIC |
|
|
|
Emulsifiers Pro's |
Longer shelf life Lower in fat |
|
|
|
Saturated fats are ________ _______ than unsaturated fats |
Saturated fats are less healthy than unsaturated fats. Saturated = animal Unsaturated = vegetable |
|
|
|
Emulsifier con's |
Allergies e.g. egg yolk |
|
|
|
Evidence for the continental drift. |
Fossils separated. Similar animals. Similar plants. Similar rocks. |
|
|
|
Earth structure from inside➡out. |
Core Inner Mantle Outer Mantle Crust Atmosphere Clever Ideas Often Challenge Assumptions |
|
|
|
Whose idea was continental drift? |

Alfred Wegener |
|
|
|
Hydrogenated oils have______ melting points |
Higher melting point. More solid. Useful as spreads. |
|
|
|
Evolution of atmosphere. Phase 1 |
Volcanoes(give off gas) thin crust. CO2. No O2. H2O. Like Mars and Venus. |
|
|
|
Evolution of atmosphere. Phase 3 |
Ozone layer O3 made by oxygen. |
|
|
|
Miller and Urey story |
Miller and Urey have a restraunt called 5 guys burger and fries. A cow in there just darted and the methane is getting on the organic matter and free range eggs. The cow is taking cover from the lightning when it orders the Primodial soup with an extra serving of hydrocarbons and amino acids. All the living organisms post this to YouTube and the story goes viral at 19:50 pm. |
Cow |
|
|
Hiw can we seperate gasses |
Fractional distillation |
|
|
|
Evolution of atmosphere. Phase 2 |
Green plants. CO2 dissolved in oceans and absorbed through photosynthesis. |
|

