![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
7 Cards in this Set
- Front
- Back
|
What is a Bohr Diagram and what does it represent? |
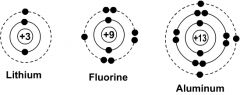
A Bohr Diagram represents the number of electrons in each shell of atoms (2 in inside one aims for 8 in the rest) |
|
|
What is an electron? |
An electron is a subatomic particle that has a negative charge of -1 and is located in the volume of atoms around the nucleus (so not in the nucleus itself) Electrons have no known components or substructure and are therefore generally considered to be elementary particles. Electrons have a negative charge of -1. Normally equal to number of protons if not it is an ion. http://www.ivyroses.com/Chemistry/GCSE/What-is-an-electron.php |
|
|
What is a proton? |
A proton is a subatomic particle found in the nucleus of atoms that differs from the other subatomic particles (called "neutrons") in the nucleus of most atoms because each proton has a positive charge of +1 (as opposed to neutrons, which have no charge). Normally equal to number of electrons if not it is an ion. http://www.ivyroses.com/Chemistry/GCSE/What-is-a-proton.php |
|
|
What is a neutron |
A neutron is a subatomic particle found in the nucleus of atoms that differs from the other subatomic particles (called "protons") in the nucleus of atoms because neutrons have no (zero) charge whereas each proton has a positive charge of +1. The number of neutrons in an atom determines which isotope of the element (which is determined by the number of protons in the atom), that particular atom is an example of. |
|
|
What is an isotope? |
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. Isotopes are atoms of the same element that have the same atomic number (whose symbol is "Z") but different mass numbers (whose symbol is "A"). Unstable isotopes are radioactive.
|
|
|
What is an ion?
|
The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. A normal atom has a neutral charge with equal numbers of positive and negative particles. That means an atom with a neutral charge is one where the number of electrons is equal to the atomic number. Ions are atoms with extra electrons or missing electrons. When you are missing an electron or two, you have a positive charge. When you have an extra electron or two, you have a negative charge. |
|
|
What is the difference between ions and isotopes?
|
Isotopes are based around the number of neutrons to the number of protons and this doesn't affect the particles charge. Whereas ions have a different number of electrons and this does affect the charge. If there is more protons it would have a positive charge. |

