![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
94 Cards in this Set
- Front
- Back
System
|
A part of the universe with set limits and consisting of one or more phases or parts.
|
|
|
Open system
|
Exchanges matter and energy with its surroundings.
|
|
|
Closed system
|
Exchanges energy, but not matter with its surroundings
|
|
|
Equilibrium
|
A system at its lowest possible energy state/level.
|
|
|
Metastable
|
A system that needs energy added to it in order to move toward equilibrium.
|
|
|
Element
|
A substance with a unique collection of properties and cannot be reduced to smaller parts without losing those properties.
|
|
|
Compound
|
A combination of one or more elements.
|
|
|
Molecule
|
A unit of matter consisting of one or more atoms.
|
|
|
Nucleus
|
Central part of an atom consisting of protons and/or neutrons.
|
|
|
Proton
|
Particle of matter carrying a positive charge
|
|
|
Neutron
|
Particle of matter without a charge.
|
|
|
Electron
|
Particle of matter carrying a negative charge.
|
|
|
Atomic number
|
The number of protons in the nucleus of an atom.
|
|
|
Atomic mass
|
The total number of particles in an atomic nucleus.
|
|
|
Electron cloud
|
The region of an atom where electrons are found.
|
|
|
Ion
|
A charged atom.
|
|
|
Cation
|
A positively charged atom.
|
|
|
Anion
|
A negatively charged atom.
|
|
|
Isotope
|
Atoms of the same element, but with differing numbers of neutrons.
|
|
|
Ionic bonds
|
Bonding of atoms caused by the transfer of electrons.
|
|
|
Covalent bonds
|
Bonding of atoms caused by the sharing of electrons.
|
|
|
Metallic bonding
|
Bonding of atoms caused by the free movement of electrons.
|
|
|
Hydrogen bonding
|
Weak attraction between polarized molecules.
|
|
|
Solid
|
Matter that is held together in rigid structure.
|
|
|
Liquid
|
Matter that is packed together in a confined space, but whose parts are in random motion relative to each other.
|
|
|
Gas
|
Matter in random motion, at high speeds, separated by empty space.
|
|
|
Plasma
|
Matter containing such high energy that the electrons have been stripped from the atoms, leaving free nuclei.
|
|
|
Mineral
|
A naturally occuring, generally inorganic, usually crystalline solid.
|
|
|
Polymorph
|
Minerals with the same chemical composition, but different internal structure.
|
|
|
Ionic substitution
|
Ionic substitution
When different elements that are similar in size and charge replace each other in the atomic structure of a mineral. |
|
|
Streak
|
The color produced from the crushed part of a mineral.
|
|
|
Luster
|
The appearance of a mineral as reflected by its surfaces.
|
|
|
Hardness
|
The degree of resistance by a mineral to scratching.
|
|
|
Cleavage
|
The tendency of a mineral to break along planes of weakness.
|
|

Conchoidal
|
When mineral fractures are curved in shape.
|
|
|
Intrusive/Plutonic
|
Igneous processes inside of the Earth.
|
|
|
Extrusive/Volcanic
|
Igneous processes outside of the Earth.
|
|
|
Vesicular
|
Texture consisting of lots of holes.
|
|
|
Pyroclastic
|
Broken, volcanic texture.
|
|
|
Fractional crystallization
|
The changing of magma composition due to crystallization.
|
|
|
Dike
|
Igneous structure of magma cutting across previous layers or features.
|
|
|
Sill
|
Structure of magma emplaced parallel to pre-existing layers.
|
|
|
Xenolith
|
A pre-existing piece of rock encased in cooled magma.
|
|
|
Aa
|
Lava consisting of broken and angular blocks.
|
|
|
Pahoehoe
|
Lava consisting of smooth or wrinkled surfaces.
|
|
|
Metamorphism
|
Chemical, mineralogical, or textural changes in a rock caused by heat and pressure, but without melting.
|
|
|
Protolith
|
Orignal rock.
|
|
|
Hydrothermal
|
Geologic processes related to hot water.
|
|
|
Foliation
|
Metamorphic texture of wrinkled or undulating surfaces caused by compression and shearing.
|
|
|
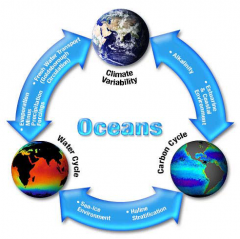
Concepts of a System
|
-Describes any part of the Universe that we wish to
examine. -It can be as small as an atom or as large as the Universe. -Consists of one or more phases or parts. •Examples: •Magma chamber •Ocean (ice, water, fish, seaweed, etc.) •Salad dressing (oil and water) |
|
|
Examples of Geologic
Systems |
The Earth is a system
•Many subsystems •Nearly closed •Small mass of meteorite and space dust input •Solar energy enters the system •Produces reactions within the system •Causes matter to flow & react |
|
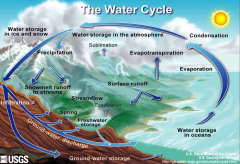
water cycle
|
w
|
|
Water cycle is open or closed system?
|
Closed
|
|
|
Ocean is a _________ system?
|
Open
|
|
|
Earth is essentially a ________ system?
|
Closed
|
|
|
Systems transfer _____ and _____ from one place to another.
|
materials and energy
|
|
|
The most stable state is always the one with the __________ energy.
|
lowest
|
|
|
Equilibrium in Geologic Systems
|
Equilibrium
• A system at its lowest possible energy level • Systems move toward equilibrium • Defining the equilibrium state determines the direction of flow |
|
|
Crystalline
|
atoms bond together in a regular orderly pattern
|
|
|
Amorphous
|
atoms bonded together in a random pattern
|
|
|
A mineral must meet the following criteria:
|
Unique crystal structure
• Atoms are arranged in a consistent and orderly geometric pattern • Homogenous – Forms through natural geological processes – Has a specific chemical composition |
|
|
Minerals grow outward from a central point and will
grow until something limits their growth: T or F |
True
|
|
|
Change in Geologic Systems
|
Flow of energy and matter
• Changes in one system component affect the entire system • Flow can be predictable because… • Systems move towards equilibrium |
|
|
The crystals grow outward
because they grow by adding atoms at the margin: T or F |
True
|
|
|
The influence of trace elements in ionic substitution can
change the color of the same mineral: T or F |
True
|
|
|
Hardness is classified using
Mohs hardness scale. T or F |
True
|
|
|
The most common minerals on Earth contain the element
silica (Si). T or F |
True
|
|
|
Quartz and Feldspar are two of the most common silicate minerals. T or F
|
True
|
|
|
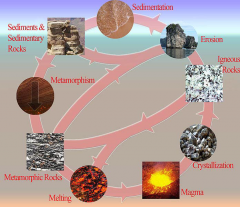
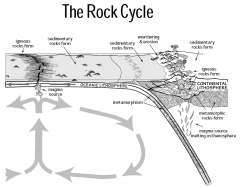
There are three main classes of rocks:
|
• Igneous – form from a melt by crystallization.
The melt is called magma • Sedimentary – formed by the accumulation of material (fragments, precipitates, or a combination of both • Metamorphic – rocks that have changed mineralogically or texturally from their original state |
|
|
tt
|
|
|
Igneous Rocks
|
• Igneous rocks form
from a melt. • They are glassy or crystalline and the crystals grow together and into each other. |
|
|
Sedimentary Rocks
|
• Formed from the broken
pieces of other rocks • Grains are held together by cement • Formed by precipitation or organic processes • They often contain fossils |
|
|
Metamorphic Rocks
|
Metamorphic rocks
are recrystallized from other rocks (even other metamorphic rocks) • Metamorphic rocks do not form from a melt, but crystals in a metamorphic rock often indicate dissolution |
|
|
Oxides: e.g. iron oxides
• Carbonates: e.g. calcite • Sulfates: e.g. gypsum • Phosphates: e.g. apatite |
True
|
|

|
True
|
|
|
Common Plutonic Rocks:
|
Peridotite – Very dense, black to green, coarsegrained
rock. Very rich in iron and magnesium (ultramafic). • Makes up most of the upper mantle of the Earth. • Gabbro and Diorite – Dark, sometimes whitespeckled rocks. • Can have a “salt and pepper” look. • Formed in magma chambers associated with ocean and continental island arcs. • Granite – Light-colored, coarse-grained rock. • Makes up most of the continental crust. |
|
|
Which of the following is a carbonate?
Calcite Apatite Stibnite Fluorite |
Calcite
|
|
|
What two types of igneous rock creates all of the oceanic crust?
sill, dike basalt, gabbro basalt, granite granite, gabbro |
basalt, gabbro
|
|
|
What is the main way that water is removed from sediments as they are altered into rock?
Decreasing pressure. Heating of the sediments. Evaporation. Compaction of the sediments. |
Compaction of the sediments.
|
|
|
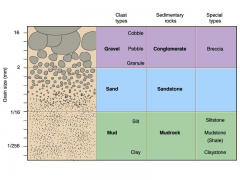
Types of Sedimentary Rocks
|
Clastic sedimentary rocks
– Most common sedimentary rock type – Form from cemented sediment grains that come from preexisting rocks • Chemical sedimentary rocks – Have crystalline textures – Form by precipitation of minerals from solution • Organic sedimentary rocks – Accumulate from remains of organisms |
|
|
Lithification
|
General term for processes converting loose sediment into
sedimentary rock – Combination of compaction and cementation – Most sedimentary rocks form from compacted and cemented grains |
|
|
true
|
|
|
Preservation
|
Sediment must be preserved, as by burial with additional
sediments, in order to become a sedimentary rock |
|
|
Clastic sedimentary rocks form where undissolved
sediment clasts are deposited either in wet (rivers, lakes, streams) or dry conditions (alluvial fans) T or F |
True
|
|
|
Breccia and Conglomerate
|
Coarse-grained clastic sedimentary rocks
– Sedimentary breccia composed of coarse, angular rock fragments cemented together – Conglomerate composed of rounded gravel cemented together |
|
|
Sandstone
|
Medium-grained clastic sedimentary rock
|
|
|
Breccia: sedimentary rocks made up of large angular
clasts |
True
|
|
|
Conglomerate: sedimentary rocks made up of
ROUNDED clasts |
True
|
|
|
Chemical Sedimentary Rocks
|
Limestone
– Limestone is composed mainly of calcite • Most are biochemical, but can be inorganic • Often contain easily recognizable fossils, coral is common • Chemical alteration of limestone in Mg-rich water solutions can produce dolomite • Chert – Hard, compact, fine-grained, formed almost entirely of silica – Can occur as layers or as lumpy nodules within other sedimentary rocks, especially limestones • Evaporites – Form from evaporating saline waters (lake, ocean) – Common examples are rock gypsum, rock salt |
|
|
Limestone is made up of the shells of animals and is
usually fossiliferous: |
True
|
|
|
Organic Material in Sedimentary Rocks
|
Coal
– Sedimentary rock forming from compaction of partially decayed plant material – Organic material deposited in water with low oxygen content • Oil and natural gas – Originate from organic matter in marine sediment – Subsurface “cooking” can change organic solids to oil and natural gas – Can accumulate in porous overlying rocks |
|
|
Features of Sedimentary Rocks
|
Sedimentary structures
– Features within sedimentary rocks produced during or just after sediment deposition – Provide clues to how and where deposition of sediments occurred – Bedding • Series of visible layers within a rock • Most common sedimentary structure – Cross-bedding • Series of thin, inclined layers within a horizontal bed of rock • Common in sandstones • Indicative of deposition in ripples, bars, dunes, deltas |
|
|
Sedimentary Structures
|
Graded bedding
– Progressive change in grain size from bottom to top of a bed. • Mud cracks – Polygonal cracks formed in drying mud. • Ripple marks – Small ridges formed on surface of sediment layer by moving wind or water. • Fossils – Traces of plants or animals preserved in rock. – Hard parts (shells, bones) more easily preserved as fossils. |
|
|
Bright colors, especially oranges, reds,
yellows, purples, and greens, usually indicate hydrothermal alteration. |
True
|

