![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
267 Cards in this Set
- Front
- Back
|
What are the 3 Reasons for Classification? |
1. Natural Desire for Order |
|
|
What system do we use for nomenclature? |
Binomial System (Genus + species) |
|
|
What are the 5 Rules for nomenclature? |
1. Only first name valid |
|
|
What is the taxonomic hierarchy? |
K - Kingdom |
|
|
What is the basis for classification? |
- Use of cladistics (grouping based off common evolutionary traits) |
|
|
What is a homologous trait? Why can they be difficult to distinguish at times? Give an example. |
- Traits which are derived from a common ancestor |
|
|
What is an analogous trait? Example? |
- A trait not derived from a common ancestor but has a similar function due to convergence |
|
|
What is a cladogram? |
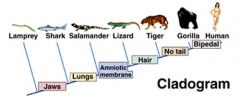
- A diagram that displays proposed evolutionary relationships among a group of species
|
|
|
3 Requirements for evolution |
1. Gene mutation |
|
|
What are the 2 essential components of a virus? |
1. Circular or linear strand of RNA or DNA |
|
|
What are the 4 features of viruses? |
1. Infectious Agents |
|
|
What is the Tobacco Mosaic Virus? |
- First Discovered Virus |
|
|
What are the 4 types of viral genome? |
1. ssRNA |
|
|
What are the 4 types of protein the viral genome codes for? |
1 type of coat protein |
|
|
Describe how retroviruses operate |
- Use reverse transcriptase to replicate |
|
|
Describe the function and structure of the viral protein capsid |
- Protects the genetic material of the virus |
|
|
What are the 3 ways a virus can enter a bacterial cell? |
1. Injection |
|
|
Describe the difference in how RNA and DNA virus cells replicate |
DNAv uses RNA Pol to create mRNAv which is then translated to viral protein whereas RNAv is directly translated. DNA viruses act within the nucleus whereas RNA viruses replicate within the cytoplasm |
|
|
What is the difference between a virion and virus? |
Virions are virus particles which act as an extracellular transport structure |
|
|
What are the 4 features which viral classification is based off? |
1. Morphology |
|
|
What is the infection and death rates of the Influenza virus? |
400 Million Infections / Year |
|
|
What is the mechanism of action for the Influenza Virus? |
There are 2 Proteins:
Each cell contains 1 gene for H and 1 for N. If there are 2 different influenza virus within the same cell, recombination of different H and N can lead to new strains. |
|
|
What drug may prevent the transmission of the Influenza Virus and how does it do this? |
Relenza is used to treat strains of influenza as it binds to Neuriminidase, preventing hydrolysis and the virus from leaving the cell |
|
|
What are viroids and how do they replicate? |
- Plant pathogens |
|
|
What are prions? What are the 2 examples of prion caused diseases and how do they occur? |
- Proteinaceous infective particles |
|
|
What are the 5 common features of the 3 Life Domains? |
1. All conduct glycolysis |
|
|
What were the 2 features of Ancient prokaryotes? |
1. They were chemoautrophic |
|
|
What are 4 features of Environmental prokaryotes? |
1. Atmospheric oxygen |
|
|
What are the differences in Bacteria vs. Archaea in terms of: |
1. Straight double bilayer with ester links vs. Branched single bilayer with ether links (lower permeability) |
|
|
What are Halobacterium halobium? |
They are prokaryotic archaea which live in hypersaline solution and use retinal as 'photosynthetic pigment' to capture light and drive chemiosmotic production of ATP |
|
|
What are 3 general features of archaea? |
1. Small Genome |
|
|
Why do bacteria have different sensitivty of toxins and antibiotics? |
They have different ribosome sizes to eukaryotes |
|
|
What are the 5 features bacterial classification is based on? |
1. Gram Stain - Positive = blue/purple = high pept content = single membrane - Negative = pink = low pept content = double membrane 2. Metabolism - Phototroph = sunlight - Lithotroph = inorganic compounds - Organotroph = organic compounds 3. Morphology - Cocci - Bacilli - Filamentous 4. Response to drugs 5. Host relationship |
|
|
What are the 5 examples of pathogenic bacterial diversity and the type of disease they can cause? |
1. Cyanobacteria (blue-green algae) - Toxic blooms 2. Spirochetes (cork shaped) - Lymes disease and syphilis 3. Gram-negative rods - Plague, cholera and salmonella 4. Actinomyocete - Tuberculosis 5. Mycoplasmas |
|
|
How do bacteria reproduce and what are the 4 ways in which they can achieve genetic diversity? |
- Reproduce through asexual binary fission 1. Conjugation - Bacterial sex which involves recombination of genome/plastid 2. Transformation - Endocytosis of dead cell genome which is incorporated in to the genome 3. Transudction - Viral DNA is combined with bacterial genome 4. Mutation - Natural chance variation |
|
|
What is the pathogenesis of a disease? |
The mechanism of infection |
|
|
What is the virulence of a disease? |
The level of infectivity of a disease |
|
|
What are 3 factors which allow for transmission of a disease? |
1. Favourable environment 2. Virulent pathogen 3. Susceptible host |
|
|
What happens at the extremes of a immune system response? |
Death occurs if immune response is overly weak or strong |
|
|
What are the 4 given examples of pathogenic bacteria? |
1. Diptheria (Corynebacterium diphtheria) 2. Tuberculosis (Mycobacterium tuberculosis) 3. Leprosy (Mycobacterium leprae) 4. Typhoid |
|
|
Where in the body does diphtheria target? What mediates it transport between cells? What is its mechanism of action? |
- Releases exotoxin in the pharynx - B subunit mediates transport between cells - Single A subunit inhibits all protein synthesis in a cell
|
|
|
What proportion of the population is affected by tuberculosis? Why is this? What is its mechanism of action?
|
- 1/3 of the population is affected as it is often in a latent form with slow division and symptom onset. - Bacteria produces mycolic acids which protect themselves against immune defense - This means macrophages are unable to kill TB and TB replicates within its vesicle, using its nutrients and causing cell death
|
|
|
What are the 4 features of leprosy in general? |
1. Unculturable human specific disease 2. Grows in macrophages of skin and nerves 3. 75% of people clear infection 4. Half of genome is pseudogenes (non-functional) |
|
|
What is the difference between tuberculoid and lepromatous leprosy? |
- Tuberculoid is the non-progressive mild form where T-Cells are able to effectively recognise antigens. It causes nerve damage and loss of sensation. - Lepromatous is the more severe form which causes granulomas in the absence of delayed hypersensitivity |
|
|
What is streptomycin? |
- Streptomycin was the first drug to be effective in treating tuberculosis - It was developed from Streptomyces griseus - It binds to the 16S ribosomal subunit |
|
|
What is the structure of fungi? (4 points) |
1. Mycelium vegetative (asexual) feeding structure 2. Cell walls composed of chitin 3. Hyphae can be divided by septa (walls) which are incomplete to allow cytoplasmic continuity 4. Hyphae can anastomose to form heterokaryons (multi-nucleate cell with genetically distinct nuclei) |
|
|
Describe the nutrition and ecology of fungi (3 points) What are the 3 ecological categories of fungus nutrition? |
1. All are heterotrophic (consume organic carbon for growth, are incapable of synthesising their own) 2. They digest food externally through enzymes 3. Energy stored as glycogen, fats and oils
3 ecological categories (saprohphytic, parasitic and mycorrhizal) |
|
|
What are saprophytic fungi and what do they consume? |
- Digest dead cell material - Decompose cellulose and lignin from plant cell wall - Major natural recyclers which can live anywhere
|
|
|
What are parasitic fungi and what do they do? |
- Form of fungus with specialised structure and nutrition - Can cause rotting, mycoses and allergies - Can be predators
|
|
|
What are mycorrhizal associations? |
- Symbiotic association between fungus and plant roots - Fungus grows in between roots and can be intracellular - Fungus receives sugars from roots and roots receive nutrients in return - 95% of plants rely on fungi |
|
|
What are the 5 types of fungal associations? |
1. Mycorrhizal (plant + fungus) 2. Endophytes (bacteria + fungus) 3. Lichens (algae + fungus) 4. Invertebrates 5. Verterbrates |
|
|
What are the 3 general stages of asexual fungal reproduction? |
Asexual => Mitosis => Spores |
|
|
What is the 6 step process of sexual reproduction which turns fungal hyphae in to spores? Explain each stage. (note 1 step has 2 alternative stages) |
Hyphae =>
1. Anastomosis (n) - fusion of 2 hyphae 2. Plasmogamy - fusion of cytoplasm 3. Heterokaryotic Stage - Cell has unfused nuclei from different mating types 4. Coenocytic Stage - No septa and multiple nuclei OR 4. Dikaryonic Stage (n+n) - 2 nuclei which do not fuse immediately 5. Karyogamy (2n) - Fusion of 2 nuclei 6. Meiosis
=spores |
|
|
What are mycoses? And why do they not occur often? |
- Mycoses are fungal diseases which are common in humans - Few fungi cause disease as they: 1. Need to grow in low oxygen 2. Have to survive the effective humman immune system 3. Have to survive at 37 Degrees |
|
|
What is the basis for mycoses classification? |
Based on taxonomy: 1. Superficial 2. Subcutaneous 3. Systemic |
|
|
What is a superficial mycoses? Example? |
A dermatophytic (infects skin) fungal disease. Epidermophyton flocossum is an example. - Causes Tinea Pedis (athletes foot) |
|
|
What are subcutaneous mycoses? Example? |
Wound and mucosa infections which spread through the lymph system. Candida albicans is an example. - Causes Thrush - Dimorphic yeast form turns in to invasive hyphae at 37 degrees - 50% incidence
|
|
|
What are systemic mycoses? What are the 2 different types of systemic mycoses? Examples of each? |
Fungal diseases which infect the entire body. 1. Invasive Opportunist - Yeast form attacks immuno-compromised individuals - E.g. Penicillum marneffei which causes penicillosis, a significant disease in AIDS patients 2. Primary Pathogen - Disease which occurs when there are high enough doses - E.g. P. brasiliensis, a wide spread disease in Central and South America which causes a progressive degredation of the lung and mouth/nose mucosa |
|
|
What is Aspergillus fumigatus and who is typically the target of its disease? |
- Invasive opportunist fungus which attacks immuno-supressed individuals (e.g. organ transplant patients) - Abundant in environment and inhalation in large numbers can cause allergic hypersensitive response known as Farmer's Lung |
|
|
What is Cryptococcus Neoformans? How does it resist attack? |
- Common and Infectious basidiomyocete yeast with over 1,000,000 cases and 500,000 deaths per year - C.gattii is an exapmle of a primary pathogen - Uses host dopamine for nutrition and melanin to help resist immune attack |
|
|
Why are fungal diseases hard to treat? What are the 4 main drugs used in antifungal therapy? |
- Hard to treat as fungal DNA is similar to humans and treatment must be target to a specific species and site of disease 1. 5-flucytosine - Mimics uracil, thereby binding and blocking nucleic acid synthesis 2. Azoles - Blocks ergosterol (similar to cholesterol) synthesis, disrupting its structure 3. Griseofulvin - Fungistatic against dermatophytes - Binds to fungal microtubules and inhibits mitosis 4. Polyenes - Disrupts membrane integriy (e.g. Nystatin) |
|
|
What are the 7 stages of fungal evolution in order of development? |
1. Protist 2. Choanoflagellate 3. Microsporidia 4. Chytridiomycota 5. Zygomycota 6. Ascomycota 7. Basidiomycota |
|
|
What are microsporidia? How do they invade cells? |
- Minute obligate parasite (needs host) - No mitochondria/flagella - Spores can be ingested by humans - Cell walls stain with calcofluor (high chitin content) - Invades cells through polar tubes which inject sporoplasm (parasite - wall)
|
|
|
What are chytridiomycota and their unique features? |
- Ancient fungi which are either soil or aquatic borne - Have motile zoospores - Hyphae are coencytic (no regular septa) |
|
|
How are zygomycota different from chytridiomycota? Describe the asexual and sexual life cycles of zygomycota. |
- Zygomycota do not have motile zoospores however are also coencytic
Asexual: reproduce by mitosis which creates sporangiospores, these are packaged within sporangium, the spore-enclosing structure
Sexual: 1. 2 zygospores (sexual) which are of a compatible mating type, anastomose. 2. These cells then undergo plasmogamy which creates a heterokaryonic (unfused nuclei) zygosporangium body with multinucleate zygospores 3. The nuclei within the zygosporangium then undergo karyogamy, forming multiple 2n spores 4. These spores undergo meiosis to become n and then germination occurs with the spores being released
|
|
|
How are ascomycota different from zygomycota? What is an example of ascomycota? Describe its asexual and sexual life cycles. |
- Ascomycota differ in that they have a dikaryon (n+n) stage and have regular septa - They have a sac-like structure - E.g. Truffles
Asexual: Hyphae form conidia (asexual spores) which fall off and join another colony or starts one on its own
Sexual: 1. 2 Ascogonium (sexual reproductive structure) fuse through plasmogamy to form a heterokaryon 2. Heterokaryon gets partitioned in to dikaryon mycelium 3. Mycelium undero karyogamy to form 2n asci 4. Asci undergo meiosis to form n asci which undergo mitosis to form an ascus with around 8 ascospores
|
|
|
How are basidiomycota different from ascomycota? What is an example of basidiomycota? Describe its sexual life cycle. |
- The asexual conidia form of reproduction is similar however it is much more rare - The Basidium (sexual reproductive structure) is club like not a sac - Similarly septate - E.g. Mushrooms
Sexual: 1. Basidium releases basidiospores (sexual spores) which can form colonies of hyphae 2. Crossing of compatible mating type hyphae leads to karyogamy forming dikaryon (n+n) hyphae. These hang as gills under the basidiocarp. 3. Karyogamy then produces 2n basidium 4. Meiosis and mitosis produces large number of genetically distinct n basidiospores. |
|
|
What are mycotoxins and its 5 main types? |
- Secondary metabolites of fungi 1. Aflatoxins - Toxic and carcinogenic - Can cause liver damage/cancer - E.g. Aspergillus fumigatus 2. Fumonosins - Common grain mould - Associated with cancer - E.g. Fusarium moniliforme 3. Ergots - Contain lysergic acid, which causes hallucinations - Cause gangrenous ergotism OR - Cause convulsive ergotism - E.g. Claviceps purpurea 4. Amatoxins - E.g. Amanita phalloides (death caps) - Cause cholera-like diarrhea 5. Tricothecene - E.g. Fusarium spp. - Livestock will not eat contaminated food |
|
|
How are fungi, as allergens, spread in outdoor vs. indoor environments. |
Outdoor: - High mould exposure in summer/autumn - Ryegrass pollen in spring
Indoor: - High humidity and condensation, low ventilation, enables growth |
|
|
How can fungi be used in drugs? Give 3 examples. |
- The metabolites of fungi can be useful in treating ailments 1. Penicilin 2. Cephalosporin 3. Anti-tumour agents |
|
|
List 4 general features of protists |
1. First eukaryotes 2. Most diverse of the 6 kingdoms 3. Mostly aquatic 4. Significant O2 source 5. Few treatment options (genetically similar) 6. Polypyletic (do not share common ancestor) |
|
|
What are the 4 structures protists all possess? |
1. Cell membrane 2. Food vacuole 3. Contractile vacuole 4. Cilia / flagella |
|
|
In order of evolutionary development, list the 6 groups of protists learnt. |
1. Diplomonad 2. Parabasilid 3. Kinetoplastid 4. Ciliate 5. Apicomplexan 6. Amoeboids
|
|
|
For diplomonads: 1. Are they flagellate? 2. Do they reproduce sexually, asexually or both? 3. Where do they inhabit? 4. What are their distinguishing features? 5. Give an example and describe 6. What treatments are available if any? |
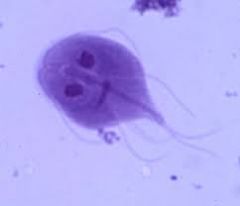
1. Flagellate 2. Asexual: trophozoite division form and cyst infective form 3. Live in water supplies and adhere to gut walls, absorbing nutrients 4. Possesses mitosome (mitochondrial remnants) which produces energy by using iron-sulfur 5. E.g. Giardia lamblia - Cause Giardiasis, also known as traveller's diarrhea 6. Treatment using 5 nitro-imidazoles |
|
|
For parabasilids: 1. Are they flagellate? 2. Do they reproduce sexually, asexually or both? 3. Where do they inhabit? 4. What are their distinguishing features? 5. Give an example and describe 6. What treatments are available if any? |
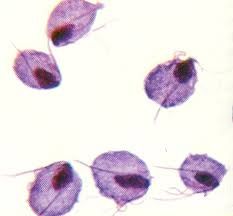
1. Flagellate 2. Reproduces asexually 3. Lives on the surface of internal organs (e.g. gut) 4. - Possess hydrogenosome instead of mitochondria, which converts pyruvate in to acetate - Has undulating membrane to assist in locomotion 5. E.g. Trichomonas vaginalis - STD which infects vagina, urethra and prostate 6. Treatment with 5 nitro-imidazoles
|
|
|
For kinetoplastids: 1. Are they flagellate? 2. Do they reproduce sexually, asexually or both? 3. Where do they inhabit? 4. What are their distinguishing features? 5. Give an example and describe 6. What treatments are available if any? |
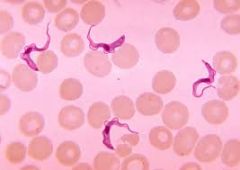
1. Flagellate 2. Asexually, but also rare sexual 3. Extracellular in blood 4. - Possess kinetoplast which is just a DNA clump - May also have undulating membrane 5. Trypanasoma (african sleeping sickness) Leishmania (leishmaniasis) 6. Prevent tsetse contact for Trypanasoma Prevent sand-fly contact for Leishmania |
|
|
For ciliates: 1. Are they flagellate? 2. Do they reproduce sexually, asexually or both? 3. Where do they inhabit? 4. What are their distinguishing features? 5. Give an example and describe 6. What treatments are available if any? |
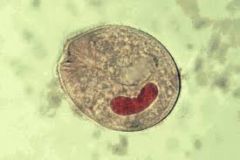
1. Small cilia 2. Both asexual and sexual 3. Inabit aquatic environments and faeces 4. i) Have 2 nuclei (macro+micro) ii) Contractile vacuole 5. E.g. Balantidium coli - Fecal transmission of cyst - Causes Balantidiasis (explosive diarrhea) 6. 5 nitro-imidazoles |
|
|
For apicomplexans: 1. Are they flagellate? 2. Do they reproduce sexually, asexually or both? 3. Where do they inhabit? 4. What are their distinguishing features? 5. Give an example and describe 6. What treatments are available if any? |
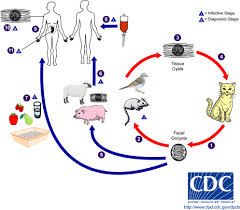
1. Immotile (no flagella) 2. Both asexual and sexual 3. Intracellular (e.g. liver cells, RBC) 4. Possess apical complex (facilitates penetration in and out of host cell) 5. E.g. Toxoplasma gondii - Cat is definitive host - Can live in the neural muscle tissue of cat, pigs and sheep - Cyst containing meat can be ingested by humans E.g. Plasmodium - Cause RBC and liver cells to rupture - Reproduce sexually on mosquito vector but human is definitive host 6. i) Prevent mosquito contact for plasmodium ii) Take prophylactic dose of Quinine => not very effective now that most strains are resistant |
|
|
For amoeboids: 1. Are they flagellate? 2. Do they reproduce sexually, asexually or both? 3. Where do they inhabit? 4. What are their distinguishing features? 5. Give an example and describe 6. What treatments are available if any? |
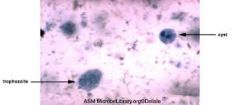
1. Pseudopodia not flagella 2. Usually asexual but rare sexual 3. Lives in stagnant fluid or bloodstream 4. i) Use pseudopodia 'feet' to move ii) No definitive shape iii) Possess contractile vacuole 5. E.g. Entamoeba histolytica/coli - Transmitted faecally through water and food - Histolytica has 4 nuclei and is the pathogenic form causing amoebiasis, engulf or lysing of RBC - Coli has 8 nuclei and is harmless 6. Prevention of faecal contamintion Use of 5 nitro-imidazoles |
|
|
What is the definition of a disease? |
An abnormal, pathological condition |
|
|
What are the 2 main groups of parasites which cause disease? |
1. Micro-parasites (e.g. virus/bacteria/fungi/protists) 2. Macro-parasites (e.g. worms/arthropods) |
|
|
Besides parasites, what is the other way disease can be caused? |
Genetic mutation or environmental stress |
|
|
What is the definition of a pathogen? |
A biological agent which causes disease |
|
|
What are the 4 stages of disease development throughout human history? |
1. Hunter Gatherer Era 2. Agricultural Revolution 3. Industrial Revolution 4. Sanitary Awakening |
|
|
How prominent was disease in the hunter gatherer era and why? |
- Disease was not very prominent 1. People lived in small isolated bands 2. They had no permenant homes so they left faeces and disease behind 3. Band members were of equal social standing, meaning less stress, better health 4. There were fewer diseases around and they had lower virulence
|
|
|
In terms of disease, what 5 factors changed between the hunter gatherer era and the agricultural revolution? What 5 pieces of evidence is their to support this? |
1. High density living 2. Animal domestication increases contacts leading to zoonoses 3. Food/water storages attracted disease vectors 4. Trading spread disease 5. People had low dietary variety
Evidence: 1. Low life expectancy 2. Parasites were recorded as being more infectious 3. Water borne diseases such as typhoid became prominent 4. Shortage of protein led to diseases such as Kwashikor 5. Dependency on agriculture meant environmental factors (e.g. droughts) led to disease and starvation
|
|
|
What 7 factors in the industrial revolution compounded the spread of disease? |
1. Even higher density of population 2. Heavy pollution 3. Skewed distribution of wealth leading to poor living conditions 4. Bedpans being emptied on sidewalks led to fecally transmitted disease 5. Water supplies contaminated with fecal matter 6. Fleas/lice were prominent vectors 7. Faster trade spread disease |
|
|
What occurred during the sanitary awakening? |
1. Chadwick observed that poor living conditions caused most disease and was thus preventable 2. London health officer employed 3. Cholera changed the opinion of the rich 4. Snow recognised water contamination as leading source of transmission 5. Aquaducts and public sewerage system + antimicrobials greatly reduced prevalence |
|
|
How does vaccination work? What different trials on smallpox led to the development of its vaccinations? |
- Vaccinations activate the immune system without causing harmful disease 1. Scratch rituals for smallpox allowed low level exposure 2. Boylston inoculated people with live smallpox => most survived the epidemic 3. Jenner vaccinates with harmless cowpox |
|
|
What are 5 present challenges, in terms of disease and biosecurity? |
1. Fast evolution of resistant strains 2. Even higher human density 3. Jet transport leads to instantaneous transmission 4. Low public awareness 5. Animal ecosystem destruction forces vectors out of natural habitat => more zoonoses |
|
|
What are the 6 requirements of a 'successful' parasite? |
1. Must survive lethal immune system attacks 2. Must survive toxins & corrosives 3. Must have slow disease => enough time to reproduce 4. Must transfer offspring safely out of the host 5. Must survive the free living stage 6. Must find another host and invade it |
|
|
What are the key aspects of small parasites (Viruses + Bacteria vs. Microbial Eukaryotes)? |
Viruses + Bacteria: - Ideal parasites due to short life cycle (rapid reproduction) and fast mutation (increased resistance)
Microbial Eukaryotes: - More complex but also have short life cycles and can achieve genetic diversity both sexually and asexually |
|
|
What are the 3 major Animalia parasite phylum and their classes? Describe each phyla in terms of their body cavity. |
1. Platyhelminthes (acoelomate) - Trematoda - Cestoidea
2. Nematoda (pseudocoelom)
3. Arthropoda (true coelom) - Hexapoda - Arachnida |
|
|
What are the 3 distinguishing features of a trematode? Example? |
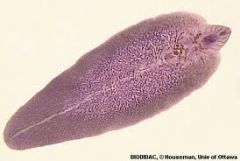
1. Suckers which act as both the mouth and anus 2. Branching gut 3. Most are hermaphroditic and composed of reproductive organs
E.g. Fasciola hepatica - Liver fluke Schistasoma |
|
|
What are the 3 distinguishing features of a cestoidea? Example? |
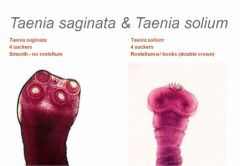
1. Scolex head with suckers and hooks 2. No gut as food is absorbed through its walls 3. Proglottid division which each contain a pair of hermaphroditic reproductive organs
E.g. Taenia saginata (cattle) Taenia solium (sheep)
|
|
|
What are the 3 distinguishing features of a nematode? Example? |
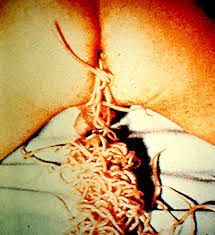
1. Ecdysozoan (molts 3 layered outer cuticle) 2. Pseudocoelomate (cavity but not mesoderm lining) 3. Not hermaphroditic (either male or female only)
E.g. Ascaris lumbricoides (inhalation) Trichinella spiralis (Meat cysts in pig and rat) Wuchereria bancrofti (Elephantitis - unchecked lymph node production)
|
|
|
Compare the main differences between Hexapoda and Arachnida |
Hexapoda = 3 body segments, 6 legs, can have antennae/wings, have spiracle breathing holes Arachnida = 2 body segment, 8 legs, no antennae/wings, have chelicerae mouths and pedipalps grabbers. |
|
|
What are 4 ways in which animalia parasites may enter the body? |
1. Through the mouth/lungs 2. Through the skin 3. Through sexual intercourse 4. Through intermediate host/vector - Eating vector - Vector scratched or carried in - Vector injected in - Vector penetrates sin |
|
|
What are the 5 survival strategies parasites may use within the body? Give examples of each |
1. Genetic variation - All 2. Hide from defender - Trichinella spiralis 3. Attack immune system - HIV 4. Present moving target - Trypanasoma 5. Avoid recognition - Schistosoma |
|
|
What are the 5 stages of parasite transmission? |
1. Inert Stage - Mechanisms allow survival in tough conditions 2. Free Living Stage - Is motile and can feed while waiting for a host 3. Intermediate Host Stage - Used as transport + helps asexual reproduction 4. Asexual Reproduction Stage - High dispersal rate 5. Sexual Reproduction Stage - Allows variation
|
|
|
Describe the life cycle of Entamoeba histolytica, including: 1. How it is transmitted 2. How it invades the body 3. How it survives immune attack 4. Where it stays 5. Its intermediate host 6. How it reproduces asexually (if at all)
|
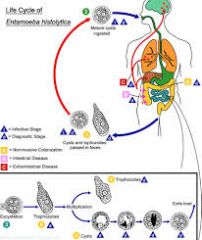
1. Transmitted from anus faeces to mouth 2. Invades through ingestion of cysts 3. No particular strategy 4. Stays in the body by boring in to the intestinal walls and reaching the vital organs 5. No intermediate host
|
|
|
Describe the life cycle of Schistosoma, including: 1. How it is transmitted 2. How it invades the body 3. How it survives immune attack 4. Where it stays 5. Its intermediate host 6. How it reproduces asexually (if at all) |
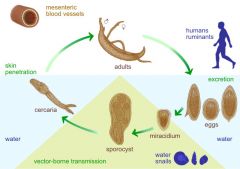
1. Transmitted through 1 intermediate host 2. Invades the body by skin penetration 3. Avoids recognition by i) Coating itself with host antigens ii) Producing complement which destroys digestive enzymes 4. Inhabits blood capillaries by puncturing gut/bladder with small thorns => dysentry 5. Snail is only intermediate host 6. Reproduces asexually on snail
|
|
|
Describe the life cycle of Echinococcus granulosus (hydatid tapeworm), including: 1. How it is transmitted 2. How it invades the body 3. How it survives immune attack 4. Where it stays 5. Its intermediate host 6. How it reproduces asexually (if at all) |

1. Transmitted through 1 intermediate host 2. Invades by ingestion of cyst meat 3. Hides from defenders by living in the gut away from the immune system. Outer cuticle = resistance to digestive enzymes. 4. Scolex attaches to intestinal wall 5. Sheep 6. Hydatid cysts can reproduce asexually in organs |
|
|
Describe the life cycle of Ascaris lumbricoides, including: 1. How it is transmitted 2. How it invades the body 3. How it survives immune attack (if any) 4. Where it stays 5. Its intermediate host (if any) 6. How it reproduces asexually (if at all) |
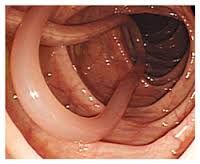
1. Transmitted from Anus faeces to mouth 2. Invades by ingestion of cysts in vegetables or inhalation 3. No particular survival strategy 4. Eggs penetrate gut => blood => lungs => throat => inhaled then returns to gut 5. No intermediate host 6. No asexual reproduction |
|
|
Describe the life cycle of Trichinella spiralis, including: 1. How it is transmitted 2. How it invades the body 3. How it survives immune attack (if any) 4. Where it stays 5. Its intermediate host (if any) 6. How it reproduces asexually (if at all) |
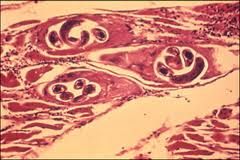
1. Transmitted through multiple intermediate hosts 2. Invades the body through ingestion of cyst meat 3. Hides from defender by living within muscle cells 4. Forms a tough cyst within muscle 5. Pig and Rat intermediate hosts 6. No asexual reproduction |
|
|
List the 8 species of pre-hominid and hominid evolution in order of development and their respective time periods |
1. Australopithecus afarensis (4-3 MYA) 2. Australopithecus africanus (3-2 MYA) 3. Australopithecus sediba (1.98 MYA) 4. Homo habilis (2 - 1.6 MYA) 5. Homo erectus (1.8 - 0.3 MYA) 6. Homo neanderthalis (300,000 - 30,000 YA) 7. Homo floriensis (12,000 YA) 8. Homo sapien (35,000 - Now) |
|
|
How has the cranial capacity developed in pre-hominids and hominids? What is the exception? What is the homo sapien cranial capacity compared to Australopithecus? |
- Cranial capacity has increased throughout time - Exception is H. floriensis (0.38L compared to neanderthalis at 1.4L) - H. sapien = 1.4 vs. Australipithecus = 0.4 - 0.5L |
|
|
How has the skull developed at each stage of hominid evolution? |

A. afarensis (ape-like) - Low & receding forehead - Prominent brow - Projecting face A. africanus - Higher forehead - Less pronounced brow - Flatter face H. habilis - Face now bigger and flatter H. erectus - Flat top - Prominent brow - Occipital ridge at rear H. neanderthalis - Deeper orbit - Modest brow - Mid face projection H. floriensis - Similar to Australopithecus H. sapien - High and domed - No brow - Rounded back - Jutting chin => complex speech |
|
|
What changes occurred to the pelvis in hominid evolution and at what species stages did they occur? |
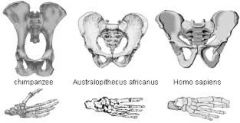
1. Intermediate and broader at A.afarensis 2. Becomes bowl-shaped at A.sediba 3. Becomes similar to modern humans (even broader) with premature births needed for large skull to fit through at H. erectus
|
|
|
How has the height of hominids changed through evolution? |
1.5m up until H. erectus when it then becomes 1.7m till H. sapiens. Exception is H. floriensis which is 1m |
|
|
How has the dentition of hominids changed through evolution? |
A.afarensis - Intermediate dentition size - Small Diastema (gaps) - Partly rounded A. africanus - No diastema - More rounded - Jaw still long H. erectus - Still has protruding jaw but is less prominent that habilis |
|
|
What are some general structural changes in the different hominid stages? |
A.afarensis - Long arms - Curled fingers (knuckle walking) - Short legs (still tree climbers) - Human-like knee A. sediba - Straight fingers H. erectus - Less hair (due to migration to north and use of furs) - Heavier skeleton |
|
|
What is Sickle Cell Anaemia? What is its pattern of inheritance? Describe its function in relation malaria |
Codomiant disease SS = Death at birth NS = Half normal haemoglobin, Half sickle cell = Immunity to malaria NN = Normal = Death due to malaria * In areas of high malaria
Single missense base change causing glutamic acid => valine
Results in defective beta globin with low oxygen affinity
NS have delayed growth, sexual immaturity and an impaired immune system |
|
|
What is Polygenic Variation? |
- When many genes determine a single trait (one gene can modify the effect of another) - New combinations can occur from sexual reproduction 'mixing' or conjugation/transposons/plasmids in bacteria - Resistant genes can be bundled with other genes to reduce bad side effects
|
|
|
How does pesticide resistance develop? |
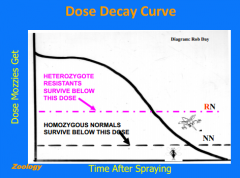
1. Very few pests with resistant gene (R) 2. All of the pests are heterozygous (RN) 3. Concentrated pesticide dose decays => There will be a period in which NN die but RN survive leading to resistance selection |
|
|
What is edge effect? |
The more edges an area has, the higher chance of pest migration. Reducing edges and treating large areas with pesticide at the same time reduces chance reinfection. |
|
|
What are 4 ways to reduce drug resistance? |
1. Have drugs with rapid decay 2. Have high drug doses with frequent and continuous use 3. Prevent unnecessary use (reduces edge effect) 4. Prevent dispersion of resistant pests |
|
|
What is horizontal gene transfer? |
- Transfer of genes through non-sexual methods - E.g. via bacteriophages or plastids |
|
|
What happens after resistance has been stabilized? |
If pesticide use continues after resistance is common: 1. Mutant genes will be retained 2. Resistant pest side effects will reduce in severity (polygenic variation bundling) 3. As most pesticides are non-specific, predators of the pests may also be killed, leading to higher pest populations (opposite effect) |
|
|
What is cross-resistance? |
Many toxins target similar aspects of insect physiology. Resistant genes can act against toxins which are similar in action as they have already adapted. Therefore, new unrelated chemicals must be used. |
|
|
What is the difference between commensals, parasites and mutualists? Give examples of each. |
Commensals - Feed off host but is tolerated - E.g. Demodex mite which is found on the base of hair follicles Parasite - Harmful effect on host and illicit negative response - E.g. Sarcoptes ectoparasite which burrows under the skin Mutualist - Beneficial to the host and illicits a positive response - E.g. Gut Flora which ferments unused substrates |
|
|
How have hosts and parasites co-evolved? |
- Parasites tend to evolve to become less harmful as harmful parasites illicit more negative responses from the host. This does not occur in areas of high population density (e.g. farms) as parasites simply find new hosts - Hosts can become resistant - Most symbionts are harmless with most of our body being covered with mutualists/commensals, the only bacteria free areas being: 1. Eyes 2. Lungs 3. Bladder |
|
|
In terms of their symbiotic relationship with humans, what are the 3 types of gut bacteria? |
1. Parasites - Use our food for their own nutrition 2. Commensals - Eat compounds we can't digest 3. Mutualists - Partially digest food for us to absorb - Feed on harmful excretions - Manufacture B&K vitamins
|
|
|
How do babies initially develop immune resistance? |
- Bifidus (vagina/nipples) decreases bacteria - Tasting behaviour allows full exposure - Diseases provide cross-over immunity |
|
|
Why are there so many harmful parasites? |
- Harmful only because they invade us by accident - Usually harmless in co-evolved hosts - Crossover is often harmful to parasite itself - Human to human transmission is rare until parasite evolves to live within human |
|
|
What is the bubonic plague and how it is transmitted? |
- A zoonosis disease which killed 1/3 people in Europe - Can be directly transmitted via coughing or through Yersina pestis (flea) vector 1. Ball of bacteria flocks flea gut 2. Blockage causes flea to move to new host 3. Recoil of sucking shoots bacteria in to new host |
|
|
What are the 3 factors which influence the virulence of a disease? |
1. Size and spatial distribution of a population 2. Whether or not there is an ongoing population of susceptibles 3. The nutrition status of a population
|
|
|
How is the rate of disease spread calculated? |
Rate of infection = r x n where n = Intrinsic rate of infection r = P x C x D P = Probability of passing with each contact C = Contacts / year D = Duration of infetiousness |
|
|
Describe the 2 different disease transmission network models. |
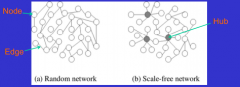
1. Random Network - Individual nodes do not typically join others 2. Scale-free Network - Hubs connected to several nodes - Higher rates of infection rates due to high standard deviation
Effective Average = Overall Average + SD |
|
|
With respect to exotic zoonoses, what can happen when an ecosystem is disrupted (e.g. due to logging)? When is zoonoses risk at its highest? |
- Exotic parasites can leave their habitat due to increased nutritional stress, thereby increasing contact with humans. - The contact between domestic and wild animals can also increase. - This spreads native pathogens. - Risk is highest when the zoonoses host is: 1. Allowed to live near a population 2. Human population is dense 3. Incubation period is long |
|
|
What are the 4 key features of rabies? |
1. 100% mortality rate once symptoms develop 2. Large RNA virus which targets the nerves 3. Disease reservoir in bats, foxes and raccoons 4. Passes to dogs then humans
|
|
|
What are 5 ways in which we can increase bio-awareness? |
1. Reduce bio-pollution 2. Immunisation 3. Public health measures 4. Minimise resistance 5. Fund bio-control research |
|
|
What must be known to develop resistance-combating drugs? |
An understanding of the pathogen's genome and its molecular action is needed |
|
|
What is chloroquin and how does it work? |
A drug which enters the digestive vacuole of plasmodium, preventing biocrystallisation of heme leading to plasmodium death. Resistant cells can pump out chloroquin. Knowledge of resistance action allows targeted drug development. |
|
|
What knowledge is useful in combating parasites? |
1. Lifecycle 2. If the parasite has predators 3. If the parasites have their own hyperparasites 4. Any other distinguishing features that can be targetted
|
|
|
Give an example of biological control through use of hyperparasites |
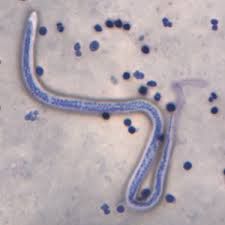
Wolbachia is a bacterium which causes the rapid replication of Wucheria bancrofti leading to elephantitis. Introducing a bacteriophage hyperparasite disrupts this. |
|
|
Why is biological control more effective than use of pesticides? Give 2 examples of predator biological control. |
- Self sustaining so reapplication is not needed however funding for initial research is often higher Examples of predator control: 1. Bacillus thuringensis (bacteria) - Cyst is made in to powder and spread across crops 2. Gambusia (i.e. instant fish) - Fish eat insects - Eggs can survive water to allow for easy spread 3. Parasitoid wasp - Can lay eggs within parasites such as the tsetse |
|
|
What are 7 features of ideal predators? |
1. Specific to prey 2. Can live in the same habitat as prey 3. Have rapid rate of reproduction 4. Easily dispersed 5. Can survive at low pest levels 6. Easily cultured 7. Do not completely wipe out pests (predators die out meaning reinfection likely) |
|
|
How would one counter a mosquito population? Relate to mosquito ecology. |
Mosquito larvae live on the water surface and can break the surface tension by using repellant tail hair. Underneath the surface, they feed and become pupae which then must once again break the water surface to emerge. By pouring oil dispersants on the water, this prevents exit, suffocating the pupae. |
|
|
What are 4 examples of non-predator biological control? |
1. Draining pools & swamps 2. Monolayer dispersants on water surface 3. Traps baited with food smell (e.g. for tsetse) 4. Tubes baited with female pheromones (e.g. for cabbage moths)
|
|
|
How does hormonal control work? |
2 types of hormones: 1. Molting hormone - Constant at each moult, induces growth of new moult 2. Juvenile hormone - Reduces each moult, at zero JH, adult forms
By providing a constant supply of JH to pests, they never reach the reproductive stage which combined with pesticide leads to an effective elimination of pests |
|
|
How would one use genetic control to reduce pest populations? What are 5 ways in which to ensure spread? Give an example. |
- Introduce 'bad' recombinant DNA DNA spread ensured by: 1. Introducing DNA for delayed sterility 2. Conditional lethality (e.g. death when cold) 3. Introducing both a gene for increased sex drive and lethality 4. Introducing meiotic drive (making transfer of one particular pair of chromosomes more favourable) 5. Introduce incompatible competing strains with 'good' genes which produce dead offspring when mating with pests
E.g. Screw Worms Recombinant DNA produces sterile offspring |
|
|
What are the 15 stages of evolution starting from reptiles, that led to homonids? Where is there a diversion in evolutionary path? |
Reptiles => 1. Mammals (class) 2. Eutheria (subclass) 3. Primates (order) 4. Plesiadapids (family) 5. Adapids (family) 6. Arthropoids (clade), seperate path leads to Prosimians (clade) 7. Tarsiers 8. Monkeys/Apes 9. New World 10.Old World 11. Gibbons 12. Orangutans 13. Chimpanzees 14. Bonobo 15. Homonids |
|
|
Name at least 6 differences in the skeleton of humans vs. apes. (9 in total) |
Humans: 1. Neck under skull not behind 3. S-shaped spine not arched 4. Bowl-shaped pelvis not long and narrow 5. Ankle bones support weight not wrists 6. Arched feet not flat 7. Femur inwards not straight 8. Straight-locking knee joint not bent 9. Forward pointing big toe not opposable |
|
|
Name at least 4 differences in the skull or cranial development of humans vs. apes. (5 in total) |
Humans: 1. 1.4L capacity not 0.4L 2. Shape of baby ape but then jaw grows more 3. Small jaw muscle => thinner, larger skull 4. APSM & HAR1 gene mutation => more control of neurons 5. Wider arteris => more glucose shunted to brain |
|
|
Name the 4 differences in the teeth of humans vs. apes. |
Humans: 1. Rounded palate 2. Small canines (smaller food items and use of tools for defense) 3. Smaller molars 4. No diastema |
|
|
What are nucleotides and its 3 components? |
Molecular units of DNA/RNA. 1. Phosphate group 2. Sugar (ribose for RNA, deoxyribose for DNA) 3. Nitrogenous base |
|
|
What are the purines vs. pyrimidines? |
Purines: 1. Adenosine 2. Guanine Pyrimidines: 1. Cytosine 2. Thymine / Uracil |
|
|
Which bases pair with each other? |
Adenosine with Thymine/Uracil Guanine with Cytosine |
|
|
Do humans have a higher GC or AT content? |
AT |
|
|
How are DNA segments packaged in to chromosomes? |
1. DNA + Histones = Nucleosome (DNA + 8 histones)
2. 6 Nucleosomes stacked = chromatin
3. Looped chromatin = chromosome |
|
|
What is the difference between euchromatin and chromatin? |
Euchromatin is the 'active' open form of chromatin which is unmethylated, allowing for transcription. Heterochromatin is methylated and has deacetylated histones, causing it to be more tightly bound and blocking transcription. |
|
|
An what basis are chromosomes karyotyped? |
1. Size 2. Centromere position i) Metacentric ii) Sub Metacentric iii) Acrocentric iv) Telocentric
|
|
|
What is the Nuclear Organiser Region? |
A region in which there is a lot of tandem rDNA which codes for rRNA sequences. |
|
|
What is the difference between aneuploidy and diploidy? Give an example of an aneuploid mutation. |
Diploidy is when you have an exact multiple of the monoploid set of chromosomes whereas aneuploidy is non-exact (e.g. Trisomy 21). |
|
|
What is Trisomy 21 and how does it occur? What determines the severity of the disease? |
A genetic mutation which causes an insertion of an extra chromosome on set 21, leading to Down's Syndrome whereby one receives mental retardation. The extra chromosomes interaction with the genome determines the severity of disease. |
|
|
What is polymorphism? |
When 2 clearly different phenotypes for the same trait exist within a population |
|
|
What is Epigenetics? Give 2 examples. |
A change in genome expression without a change in the structure of DNA. E.g. 1. Histone methylation (euchromatin vs. hetero) 2. Genomic imprinting |
|
|
What are the 3 variations in how phenotypes are influenced? Give examples of each. |
1. Environmental factors E.g. Fetal Alcohol Syndrome - Mother drinking causes damage to fetus and growth abnormalities 2. Genetic factors E.g. Huntington's Disease - Excessive repeat of CAG triplet 3. A combination of environment + genes E.g. Curly Wing - Gene only expressed in high temperatures
|
|
|
What is mosaicism? How does it occur? |
When cells in the same person has different genetic makeup. Can be caused in heterozygous females due to X-inactivation. Can also occur due to somatic mutation. |
|
|
How does Klinefelter's Syndrome occur? What is its prevalence in society? Name 2 symptoms. |
Occurs due to an extra X chromosome insertion => XXY 1/500 have it 1. Small testes => reduced testosterone 2. Breast enlargement 3. Infertility |
|
|
Why is aneuploidy risk higher if a mother is at a higher age? |
Cohesin which binds sister chromatids together in gametes does not function properly at older ages leading to non-disjunction at anaphase of meiosis or mitosis (mosaic). |
|
|
What is polyploidy? |
When there is more than 2 sets of chromosomes (e.g 3N) |
|
|
What are the 6 methods for testing chromosomal abnormalities? |
1. Ultrasound - Look at nuchal fold to determine Down's 2. Pre-implantation Diagnosis - Biopsy of 1 undifferentiated embryo cell 3. Maternal Blood Triple Test Test for i) AFP - alpha fetoprotein produce in the amniotic sac ii) hCG - human chorionic gonadotropic iii) Oestriol - estrogen produced by the fetus + placenta 4. Cell Free DNA -Dead fetal cells in the blood stream (noninvasive) 5. Chonionic Villus Sampling - Sample of chorion membrane in between the fetus and mother (invasive) 6. Amniocentosis - Amniotic fluid swallowed and excreted by fetus, fetal cells seperated and diagnosed for down's and neural tube |
|
|
Give the 8 stages through which DNA replication occurs (Not PCR) |
Occurs in the synthesis stage of mitosis 1. DNA Topoisomerase unwinds the double helix 2. Helicase breaks H bond 3. Nucleoside Stabilising Protein keeps the strands apart 4. RNA Primer set as DNA replication must start from a double strand 5. DNA Pol 2 - Adds complementary bases from 5' to 3', starting at the 3' end of the leading strand 6. Multiple RNA Primers needed as 3' on the lagging stranded in only gradually revealed. Once copying hits an old primer, the primer falls off leading to Okazaki Fragments 7. DNA Pol 1 fills in the gaps in between Okazaki 8. Ligase joins fragments |
|
|
What are 4 differences between eukaryotes and prokaryotes in terms of DNA replication? |
Eukaryotes: 1. 100-200 bases not 1000 2. Several DNA polymerases not only 3 and 1 3. 3000 bases per minute not 30000 bases 4. Nucleosomes disassembled |
|
|
What are 3 ways the body can repair its DNA? |
1. Proofreading mechanisms - Replication complex protein immediately excises incorrect nucleotides - DNA pol adds correct 2. Mismatch repair - Missed by proofreading, excises mismatched + adjacent - DNA pol adds correct and ligase binds 3. Excision Repair - Same as mismatch but with damaged nucleotides not mismatched |
|
|
What are the 5 ingredients of PCR? |
1. DNA 2. Deoxyribosenucleotide triphosphates 3. Enzymes (taq polymerase from Thermus aquaticus) 4. Primers 5. Buffers |
|
|
What are the 3 steps of PCR and what happens at each? |
1. Denaturation - Seperate DNA strands through temperature increase or alkali 2. Annealing - Decrease temperture - Add primer bases 3. Extension - Polymerase extends strands
|
|
|
What is the origin of replication? |
The point at which DNA replication starts |
|
|
What are telomeres? |
- Repeated nucleotide sequences (TTAGGG) at the end of chromosomes usually at around 2500 repeats - Protect the end by looping/recruiting protective proteins - Lagging strand often has incomplete okazakis leading to gradual loss of telomeres (50-200) each replication |
|
|
What is telomerase? |
An enzyme which extends the lagging strand telomere by completing replication of the last okazaki strand. Contains and RNA counterpart which adds bases. |
|
|
What do genes code for? What were the 2 original theories and how are they inaccurate? |
Genes code for polypeptides and RNA. Not enzymes as different proteins also coded for. Not just proteins as protein subunits can be coded from different genes. |
|
|
What is Alkaptonuria? |
Tyrosine usually produces homogentistic acid which is converted to maleylacetoacetic acid by and enzyme. Alkaptonuria is a defect in this enzyme, causing an acummlation of homogentistic acid. |
|
|
What did the Beedle and Tatum experiment test? |
1. Neurospora spores irradiated 2. Wild types are prototrophs and can survive on minimal media 3. Once irradiated, they become auxotrophs which require supplements to survive on minimal media 4. By placing different supplements and determining which mutants will grow, it can be determined that a single gene codes for 1 enzyme |
|
|
What is codominance? Give an example |
When the full effects of both alleles can be seen in a heterozygote individual. E.g. Sickle Cell Anemia |
|
|
What is Incomplete Dominance? Give an example |
When the heterozygous allele individual has a completely different phenotype from the homozygotes. E.g. Snapdragon. |
|
|
How many different genotypes and phenotypes would 3 alleles at a single gene locus produce? |
3 Phenotypes 6 Genotypes |
|
|
What is pleiotropy? What is an example? |
1 Gene affecting many features of the phenotype E.g. Sickle Cell |
|
|
What is a phenocopy? Example? |
When environmental factors can mimic the phenotype of an inherited condition? Height is dependent on genetic factors but can also be influenced by environmental factors such as nutrition |
|
|
What is gene expressivity? Example? |
When there is a variable range of phenotypic expression for an allele. E.g. Polydactly |
|
|
What is gene Penetrance? |
The proportion of people in a population with a given genotype that actually expresses the phenotype. Reduced penetrance occurs when an individual shows incomplete expression of a trait.
|
|
|
What is lethality? Example? |
Dominant single allele = death E.g. Huntington's - delayed onset
Recessive double allele = death |
|
|
What are the 3 systems of sex related inheritance? Give examples of 2 animal groups which use each. |
1. XY = Male (heterogametic), XX = Female (homogametic) - Mammals, Drosophila
2. ZZ = Male (heterogametic), ZW = Female (homogametic) - Birds, Fish, Butterflies, Reptiles
3. XO = Male, XX = Female - Crickets, Roaches, Other Insects
|
|
|
What are the 5 Sex-Determination mechanisms? |
1. Environment 2. Autosomal Genes 3. Haploidy/Diploidy 4. Balance of Sex/Auto Chromosomes 5. Sex chromosomes |
|
|
Give 2 examples of how sex can be determined by the environment. |
1. Bonella viridis is male if offspring lands near the probiscus as there are chemicals which determine sex there 2. Turtles will be male at low temperatures 3. Lizards will be male at high temperatures
|
|
|
What are 2 ways in which the autosomal genotype can determine an individuals sex? |
1. double tra recessive mutation causes XX individuals become male 2. A *tfm mutation causes receptors in tissues to become insensitive to testosterone, this leads to androgen insensitivity, causing XY individuals to become feminine (outwardly female but no internal reproductive organs + undescended testes)
|
|
|
How can being haploid vs. diploid affect sex? |
In bees, sex depends on cell ploidy. N = Male Drone 2N = Female |
|
|
How does the balance of sex/autosomal chromosomes determine sex in drosophila? |
For drosophila: X/A = 1 = female, X/A > 1 = meta female (sterile) X/A = 0.5 = male, X/A <0.5 = meta male |
|
|
What are 2 examples in which abnormal amounts of sex chromosomes can determine sex? |
1. Turner's Syndrome - Aneuploidy = XO - 70-80% result from sperm losing sex chromosome - Female with underdeveloped uterus 2. Klinefelter's - Aneuploidy = XXY - Non-disjunction of sperm in anaphase 1: XY + X
|
|
|
What are the 3 groups of genes on the human Y chromosome? |
1. SRY (sex-determining region Y which includes TDF) 2. Testis specific expression genes 3. Housekeeping genes |
|
|
What part of the Y chromosome is homologous in the X chromosome? |
The pseudoautosomal region which can cross over with similar regions in the other sex chromosome |
|
|
What is TDF and why is sometimes mistakenly found on X chromosomes? What happens? |
- Gene transcription factor which activates SOX9 - Allows differentiation of seminiferous tubules TDF is close to the pseudautosomal region, hence crossing over sometimes causes it to be accidentally synapsed on the the X chromosome. This causes development of Testes. |
|
|
How do male reproductive organs develop? |
XY => TDF => Testes => AMH + Testosterone AMH = No mullerian development = No internal female genitalia Testosterone = Wolfiann development (internal) + dihydrotestosterone (external)
|
|
|
How do female reproductive organs develop? |
XX => Ovaries + No AMH Ovaries => Oestradiol => external genitalia No AMH => Mullerian development => internal |
|
|
What is 5 alpha-reductase? What does deficiency lead to? |
- Enzyme which converts testosterone to dihydrotestosterone - Deficiency leads to pseudohermaphroditism meaning no external male genitalia - Puberty may lead to sufficient levels of dihydro/testosterone meaning females can become males |
|
|
What is true hermaphroditism? How does it occur? |
When an individual has both functioning testes and uterus. Can occur when XX have Y derived sequences or due to XX/XY mosaicism. |
|
|
What are the 3 types of sex linkage? Give examples of each where possible. |
1. X - Affect males more than females - E.g. Haemophelia 2. Y - Passed from father => son - E.g. TDF => androgen insesitivity 3. Z - Male is homozygous ZZ - Female is heterozygous ZW - Recessive traits passed from father to daughter |
|
|
What is X-Inactivation (also known as lyonisation) and how does it occur? How many barr bodies are produced? |
1. XIST on the XIC produces RNAi which coats all but 1 X chromosome in females. 2. This causes these chromosomes to be packaged in to transcriptionally inactive heterochromatin
No. of Barr bodies = X(n-1)
|
|
|
Why are females who are heterozygous for X-linked traits, mosaics? |
Lyonisation causes one of the two alleles to be inactivated, hence cells will have 50:50 of the alleles. |
|
|
Why aren't all X chromosomes inactivated by XIST? |
Active chromosomes have TSIX gene which blocks activation of XIST. |
|
|
If X-Inactivation occurs, what are 2 reasons individuals with an abnormal number of X chromosomes still show symptoms? |
1. Inactivation doesn't occur till after the 500 embryo cell stage, hence symptoms can manifest before then 2. Genes from the pseudoautosomal regions of extra X chromosomes are still transcribed. |
|
|
What is atypical lyonisation? |
Occurs when heterozygous females show a skewed inactivation of a certain allele through chance. |
|
|
What are sex influenced traits? Provide 2 examples. |
When autosomal gene expression is influenced by sex. 1. Male pattern baldness - Overexpression of 5 alpha-reductase causes hair loss in males 2. Polycystic Ovary Disease (PCOD) - acne facial hair on females |
|
|
What are sex-limited traits? Provide 2 Examples. |
When phenotypic expression is limited to a single sex. 1. Male Precocious puberty - Not possible in females due to hormonal profile 2. Testicular Feminisation Mutation (TFM) - Has no extra effect on females as they do not need testosterone receptors |
|
|
As a function of the number of chromosome pairs, what are possible number of gamete combinations? |
Possible gametes = 2^(chromosomes) E.g. Aa;Bb;Bc Can produce 2^3 = 8 possible gametes |
|
|
Assuming independent assortment, what is the phenotypic ratio for an autosomal dihybrid cross involving 2 heterozygous chromosomes?
|
9:3:3:1 |
|
|
What are the 3 types of diagnostic crosses? What are they each used for? |
1. Test - Cross of a dominant phenotype individual to homo recessive to determine if it is homo dominant or heterozygous 2. Reciprocal - Switch phenotype of purebreeding parents to other sex - Autosomal or X-Linked 3. Back - Crossing of F1 with purebreed parents - Used to maintain homozygosity of desirable traits, usually used in agriculture
|
|
|
What is Gene Interaction? |
When one or more genes interact to affect the same phenotype. E.g. Locus 1 = determines colour of coat Locus 2 = density of colour of coat Secondary genes are called modifier genes |
|
|
What are the 5 types of epistasis? What are their respective phenotypic ratios? |
1. Recessive 9:3:4 2. Dominant 12:3:1 3. Duplicate Gene Action - Either enzyme can produce precursor (hence only aa;bb doesn't produce end phenotype) 15:1 4. Complementary Gene Action - Both genes required to produce end phenotype (A-B-) 9:7 5. Dominant suppression - Homo recessive can produce end phenotype 13:3 |
|
|
What is epistasis? Give an example. |
A special form of gene interaction whereby one gene masks the phenotypic expression of another. Mice colour: aa gene produces enzyme which allows albino => black BB and Bb genes produces enzyme which allows black => agouti Even if BB/Bb is present, if aa is not, the mice will still be albino This is an example of recessive epistasis |
|
|
What is genetic heterogenity? Why does this mean 2 infected individuals for an auto recessive trait can produce unaffected children? |
When 2 different genes code for the same phenotype. E.g. Both A and B cause ailment aa;BB x AA;bb Produce Aa;Bb offspring without ailment |
|
|
What pattern of inheritance do ABO blood groups follow? Explain. |
Codominance 3 completely different phenotypes A- = A antigen, B antibodies AB = Both A and B antigens, No antigens B- = B antigen, A antibodies O = No antigen, AB antibodies |
|
|
What is the bombay phenotype? What is it an example of? |
A phenotype caused by homozygous recessive h alleles => no production of H carbohydrate used to produce Antigen A, B => AB antibodies => phenotypically similar to O Example of recessive epistasis as hh supresses the antigen phenotypes |
|
|
Explain the rhesus blood grouping system. How does haemolytic disease of the newborn occur? |
2 Alleles (D and d) Rh +ve D is dominant and codes for D antigen D antibodies produced in response to D antigen presence, only in dd. A placenta which is Dd can transfer blood to the mother, causing exposure to the D antigen, thereby producing a D antibody response. Future children are then killed because the D antibodies attack them. |
|
|
How may haemolytic disease of the newborn be treated? |
Using RhoGAM, provides antibodies to kill initial antigens, thereby stopping immune response |
|
|
What is linkage analysis? |
The testing of one loci, by testing for the other which is linked |
|
|
What is the formula used to find interference? What does a positive/negative number infer? |
Interference = 1 - CC CC = Observersed/Expected Expected = distance 1 x distance 2 x total freq.
Positive means one crossover inhibits the other Negative means one crossover promotoes the other |
|
|
What are the 2 forms of polygenic inheritance? |
1. Discontinuous - Clear cut presence of non-presence of a phenotype i) One gene - (e.g. ABO) ii) Multiple genes + environment - (e.g. NTD where gene influence + accumulative environmental factors lead to tipping of threshold) 2. Continuous - Qualitative - Controlled by many gene loci - No dominance |
|
|
How do you calculate the lowest polygene frequency? |
Lowest frequency = (1/4)^n Where n = the number of polygenes |
|
|
What is the difference between monozygous and dizygous twins? Why are they useful for genetic analysis of traits? |
Monoyzgous: Share same placenta and are genetically identical at birth
Dizygous: 2 eggs fertilized seperately and share seperate placenta (essentially, brothers/sisters)
Useful as both Monozygous twins are subject to the same genes and environmental influence whereas dizygous twins are only subject to environmental influence, hence comparison of the two can be used to determine gene influence. |
|
|
What is heritability? |
A measure of how strongly the phenotypes of offspring match the parents |
|
|
How would one determine the degree of genetic influence? |
Measure concordance (if both twins have trait) for monozygous and dizygous pairs. Genetic influence = MZ - DZ |
|
|
What are the 3 steps in Gene Transcription (eukaryotes) and describe what happens in each. |
1. Initiation i) Transcription factors bind to the promoter region ii) RNA pol also binds to promoter iii) Proteins can bind to enhancer region, allowing it to bind to the promoter, thereby starting elongation iv) Elongation factor drops off 2. Elongation and termination i) Bases enter the RNA pol and create a new mRNA strand from 5' to 3' as it travels down the coding region from 3' to 5' ii) This happens until the RNA Pol hits a termination sequence, which forms a loop allowing the mRNA to drop off 3. Processing (eukaryotes) i) MeG Cap added to allow ribosomal binding to mRNA ii) Poly A Tail added for stability |
|
|
How does splicing occur? What is alternative splicing? |
1. SnRNA binds to the splicing site between exon and intron junctinos 2. Interactions between snRNA on either side of the intron to form a lariat, leading to what is a splicosome 3. This results in the formation of mature RNA
Alternative splicing occurs when exons adjacent to introns are cut out. This is a regulated process which allows a single primary transcript to code for multiple polypeptides. |
|
|
Name 3 differences in Eukaryotic vs. Prokaryotic Transcription |
Eukaryotic: 1. Multiple RNA Polymerases, not single 2. Primary transcript incomplete 3. 3 Types of promoters: i) Pol 1: rRNA ii) Pol 2: mRNA, RNAi iii) Pol 3: tRNA, snRNA |
|
|
What is a holoenzyme? |
The transcription factor which is used in prokaryotes. 5 subunits which bind, then sigma subunit comes to allow elongation to start. Sigma subunit dissociates after. |
|
|
How many genes in the human genome code for polypeptides? RNA? |
Polypeptides: 20,000 RNA: 5000-6000 |
|
|
What is the average gene size for a DNA and mRNA segment in the human genome? |
DNA: 25,000 nucleotide base pairs mRNA: 3000 nucleotides |
|
|
What are RNAi? What is the difference between its two types? |
RNA molecules which inhibit gene expression microRNA do not need to be fully complementary to mRNA in order to degrade, whereas siRNA need to be. |
|
|
How many different possible codon based combinations are there? Why are there only 20 essential amino acids? |
- 4^3 = 64 - Only 20 due to wobble and some being start and stop codons |
|
|
What are the start and stop codons? |
Start: AUG
Stop: UAA UAG UGA |
|
|
What is the shine-delgano sequence? (Not the actual sequence itself obviously) |
The sequence of the mRNA where the small ribosomal unit can bind in order to allow translation to start. |
|
|
What are the 2 components of a charged tRNA (aminoacyl-tRNA)? |
1. Uncharged tRNA anticodon 2. Complementary amino acid
|
|
|
Describe the 3 steps through which mRNA is translated in to Protein |
1. Initiation - Small ribosomal unit binds to shine - F-met enters in the p-position on ribosome 2. Elongation - Charged tRNA attaches to aminoacyl binding site - Uncharged tRNA leaves via the E-site - Peptide bond formed between the amino acids in the P and A binding sites 3. Termination - At stop codon, a termination protein attaches, causing protein to drop off
|
|
|
What are 3 ways in which Gene Expression can be regulated? |
1. Chromatin structure - Methylation of cytosines 2. Transcriptional regulation - Environment (e.g. GFP arabinose dependence) - Enhancers, repressors (e.g. RNAi) 3. Post-translation regulation - E.g. protein phosphorylation
|
|
|
What are chromosomal mutations? List 2 types |
Gross mutation that involve several genes 1) Inversions i) Paracentric (non centromere) ii) Pericentric (centromere) 2) Translocation - Same genetical material but on different chromosomes - Can lead to new gene products |
|
|
What are the 3 types of base substitution mutations? |
1. Silent - Switch in single base in the wobble => same genetic amino acid - Can affect rate of translation 2. Missense - Switch in single amino acid (e.g. sickle cell) 3. Nonsense - Switch to termination codon - More severe if early in the sequence
|
|
|
What is a frameshift? |
When bases are added/deleted in non-multiples of 3, leading to the code reading frame being out of phase. E.g. Becker's |
|
|
What is the pattern of inheritance for cystic fibrosis? What is it caused by and what does it cause? |
Hetero recessive disorder caused by delta F508 (deletion of phenylalanine on position 508) leading to an in-frame deletion of the CFTR protein gene responsible for chloride ion transport => accumulation of sticky mucous |
|
|
What is Haemophelia A and its pattern of inheritance? What is it caused by and what does it result in? |
An X-linked recessive disorder. Causes a deficiency in clotting factor VIII. This is needed in thrombin and subsequent fibrin production, a lack meaning blood cannot properly clot. |
|
|
Why are symptoms of beta thalassaemia typically only seen in 6-12 months after birth? |
Fetal haemoglobin (Hb) contains gamma units instead of beta, hence a persistence of fetal haemoglobin after birth means defect in beta globin has less effect. |
|
|
What is the pattern of inheritance for alpha and beta thalassemia? How can alpha thalassaemia occur? |
-Auto recessive disorders which lead to decreased stability or synthesis of respective globin genes - Alpha thalessaemia can be the result of uneven crossover (non-alignment of homologous chromosomes), with two or less gene units typically resulting in disease. |
|
|
What is the pattern of inheritance for Huntington's Disease? What is it caused by and what does it cause? |
- Auto dominant pattern of inheritance with lethality (delayed onset) - Caused by CAG triplet repeat in the coding sequence => gain of function => production of Huntington protein => accumulation causes neurological disease - Severity inversely proportional to age |
|
|
What is Fragile-X syndrome? What is its pattern of inheritance? What is it caused by and what does it cause? |
- X-linked disease - Caused by expanded CGG triple repeat in the 5' UTR - If repeat is large enough, promoter region remains untranscribed, leading to loss of coding for FMRP (fragile-X mental retardation protein) which is required for normal neuron function - One of the most common causes for mental retardation - Premutations are amplified by passage through females |
|
|
Explain the process of southern blotting |
1. DNA cut by restriction enzyme endonucleases 2. Separated by size using electrophoresis 3. Blotted on to filter 4. DNA denatured 5. Hybridised with probes specific to DNA sequence in question, plus a label (e.g. fluorescence) 6. Allowed to renature 7. It can be determined how far the DNA in question has travelled
|
|
|
What are restriction enzymes? What are the two types? |
-Endonucleases produced by bacteria which make double stranded cuts in the DNA I - Cuts at a specific base length away from recognition sequence II - Cuts at a specific sequence |
|
|
What are sticky ends? |
A staggered 5' and 3' end of dsDNA caused by restriction enzyme cutting. These ends are complementary to other 3' and 5' ends. |
|
|
How can molecular cloning be achieved through a cloning vector? Use an example. |
E.g. - Insert plasmid with LacZ gene in to bacteria - Cut plasmid with restriction enzyme targeted at LacZ sequence thereby disrupting it - Add human DNA - Plasmid can either close => blue colonies who have a functioning LacZ gene - Or it can take up the human DNA, disrupting the LacZ which is required for lactose metabolism, resulting in white colonies - Selectively take out the white colonies as these ones have the human DNA for cloning |
|
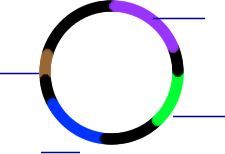
Label this plz.
|
Starting from brown segment in clockwise: 1. OriC - origin of replication 2. AraC - regulatory protein that enhances promotion in presence of arabinose 3. GFP - protein that fluoresces under UV if transcribed 4. Beta lactamase - Ampicillin resistance I.e. If bacteria uptake this plasmid, + arabinose is present, they will be ampicillin resistant and will glow under UV |
|
|
How can triploidy be used as evidence for genomic imprinting? |
2N Mat + 1N Pat = Normal fetus development 2N Pat + 1N Mat = Teratoma This suggests that maternal genes are genomically imprinted as having 2 mat provides a normal fetal development (i.e. gene suppressed) |
|
|
Explain the difference between Prader Willi and Angelman's Syndrome. |
Both are caused by deletions in the same region of chromosome 15. Difference is caused by genomic imprinting. Paternal deletion = prader willi = Low IQ, High appetite Maternal deletion = angel man = puppet-like movement |
|
|
If 60% of Prader Willi is caused by genomic imprinting, what causes the other 40% of cases. |
Uniparental disomy: *Incoming gamete is usually ejected to prevent triploidy Heterodisomy: Caused by non-disjunction at anaphase I, leads to genetically similar gamete as parent Isodisomy: Caused by non-disjunction at anaphase 2, leads to 2 sets of 1 chromosome from parent, can lead to the duplication of lethal recessive or 2 non-imprinted or imprinted genes. |
|
|
What is cancer? |
A clone of cells diving in an unregulated way as a result of somatic or hereditary mutations. |
|
|
What is the difference between a sarcoma and a carcinoma? |
Sarcanoma = mesodermal cancer Carcinoma = epithelial cancer |
|
|
Why does cancer have a delayed onset? |
Develops with age as it is an accumulation of somatic mutations |
|
|
What is the difference between malignant and benign tumours? |
Malignant are metastatic and can spread to other areas of the body. |
|
|
What is the proto-oncogene normally responsible for? |
Regulated cell growth and proliferation |
|
|
What are 2 steps in which cancer can develop? |
1. Loss of TSG gene due to mutation 2. Proto-oncogene mutation in to oncogen which cannot be regulated by TSG |
|
|
What is Burkitt's lymphoma? |
A translocation of an enhancer on Chr 14 next to a proto-oncogen on Chr 8, leading to the formation of an oncogene causing cancer. |
|
|
What is the role of the Tumour Suppressor Gene? When does is stop being effective? How may this occur? |
Regulates cell proliferation. Both copies of gene must be knocked out for it to stop functioning. Can occur via: 1. Deletion 2. Mutation 3. Epigenetic Effect |
|
|
How does cancer progress to a fully developed form? |
Involves many genes and mutations, is not the result of a single step |
|
|
What are 7 ways in which genetic variation may be detected? |
1. Visible phenotype difference 2. Chromosome difference (e.g. Y arm length) 3. Immunological markers (e.g. ABO) 4. Protein Gel Electrophoresis distances 5. VNTR Probes 6. STR Analysis 7. Single Nucleotide Polymorphisms |
|
|
What are variable number tandem repeats? |
-Repeat sequences in non-coding regions of between 15-100b -No. of repeats different in people (polymorphic) -Often replicated in may different loci, hence single probe sequence can determine the size of the DNA on which repeats are found => unique profile
|
|
|
What are Short Tandem Repeats? |
-Repeat sequences of between 2-9 b -Several alleles depending on number of repeats -Easier to analyse for DNA profiling because can be easily amplified by PCR but there are few variations in profile => must simultaneously test a large no. of profiles |
|
|
What are Single Nucleotide Polymorphisms? |
Differences in single bases in the genome (e.g. through insertion/deletion) - Can be detected through microarrays - Has to be present in more than 1% of the population to be considered a SNP |
|
|
What are the 5 assumptions of Hardy-Weinburg? |
1. Random Mating 2. No Migration 3. No Selection 4. No Mutation 5. Infinite Population Size |

