![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
155 Cards in this Set
- Front
- Back
|
Describe the HPV virus. |
HPV is a DNA virus which infects keratinocytes of the skin and mucous membranes. Over 170 types (strains) of HPV have been identified to date; most strains infect the anogenital region. |
|
|
HPV has been tied to which cancer types? |
1. Oral squamous cell carcinoma (rare) 2. Oropharyngeal squamous cell carcinoma 3. Cervical cancer |
|
|
Which HPV viral proteins contribute to carcinogenesis and how? |
-E5 modulates EGFR expression by inhibiting the internalization and degradation of the receptor leading to increased EGFR-induced cell proliferation -E6 is an ubiquitin ligase that targets p53 for proteasomal degradation, thereby compromising p53-dependent cell-cycle arrest and apoptosis -E7 binds and inhibits retinoblastoma, leading to increase activity of EF2 (TF, activates genes involved in cell cycle progression) and increased expression of p16 (a CDK inhibitor) |
|
|
Oral cancer affects what parts of the body? (6) |
-Tongue -Floor of the mouth -Hard palate -Buccal mucosa -Alveolar ridge -Lip |
|
|
What are the most common risk factors for developing oral cancer? |
- Smoking -Alcohol consumption -HPV (oral HPV is likely spread through oral-genital contact; its role in OSCC is not as well understood as for OPSCC or cervical cancer) -UV exposure (increased risk of lip cancer) -Chronic irritation of the tongue, e.g., dental cavities, overuse of mouthwash, chewing tobacco, betel quid (plant-based stimulant common in India) |
|
|
What biomarkers exist for oral cancer? |
Elevated levels of interleukin-8 and SAT (Elashoff et al., 2012) |
|
|
Characteristics of SCC-4 (1624) cells. |
-Tongue -55 yrs, male -stage T3N0M0 -patient had radiation and methotrexate treatment for 16 months prior to biopsy -Epithelial cell morphology -Weak colonies |
|
|
What is sphingosine? |
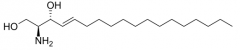
Sphingosine (2-amino-4-octadecene-1,3-diol) is an 18-C amino alcohol with an unsaturated hydrocarbon chain which forms a primary part of sphingolipids, including sphingomyelin |
|
|
Describe sphingosine phosphorylation. What proteins are involved and how do they differ? |
Sphingosine phosphorylation creates spingosine-1-phosphate, a potent lipid second messenger. It can be phosphorylated by sphingosine kinase(SphK) 1 (which is localized to the cytosol and migrates to the plasma membrane upon activation) or Sphk2 (which is localized to the nucleus) |
|
|
What is sphingomyelin? Describe its structure. |
Sphingomyelinis a type of sphingolipid found in animal cell membranes. Composition: A phosphocholine head group, a sphingosine, and a fatty acid (sphingosine + fatty acid = ceramide) |
|
|
What are ceramides? |
Ceramides are a family of lipid molecules found in cell membranes. They are a componentof sphingomyelin, and form an important part of many biological signaling pathways |
|
|
Describe the structure of a ceramide. |
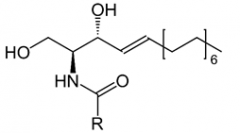
Ceramides are composed of a sphingosine and a fatty acid. The R group denotes the alkyl portion of a fatty acid. |
|
|
What is smpd3? |
Smpd3, or sphingomyelin phosphodiesterase 3, is a human gene ecnoding the neutral sphingomyelinase 2 (nSMase 2) protein. |
|
|
What are the functions of nSMase2? |
nSMase2 hydrolyzes the phosphodiester bond of sphingomyelin to yield phosphocholine and a ceramide. |
|
|
Name the most commonly used chemical inhibitor of nSMase2. How does it work? |
GW4869. It acts as a noncompetitive inhibitor. |
|
|
What are exosomes? |
Exosomes are small, membrane-bound vesicles generally considered to be between 50-120 nm diameter. |
|
|
Do exosome markers exist? |
Exosomes are known to contain the tetraspanin CD63 in their membrane, though this protein is not specific to exosomes. |
|
|
Describe the events which take place on the outer and inner leaves of the membrane of a MVB during exosome budding. How do these events contribute to budding? |
-Outer (cytosolic) leaflet: nSmase converts sphingomyelin to phosphocholine and ceramide -Inner (luminal) leaflet: Group V secretory phospholipase A2 generates lyso-phosphatidylcholine (lyso-PC) and lyso-phosphatidylethanolamine (lyso-PE) -The cone-like shape of ceramide in the outer leaflet and the inverted cone-like shape of lyso-PC and lyso-PE as well as glycosphingolipids in the inner leaflet induce membrane curvature by creating an area difference between the leaflets. |
|
|
Oropharyngeal squamous cell carcinoma affects which parts of the body? |
-Soft palate -Posterior pharyngeal wall -Palatine tonsil -Lingual tonsil |
|
|
What are the common sites of oral cancer metastasis? |
The most common site is the lymph nodes of the neck, collectively called the cervical lymph nodes. From there, cells may spread further, most commonly to the lung. Brain, bone, skin, and (very rarely) abdominal wall metastases have been reported. |
|
|
Describe the chracteristics of Cal27 (2095) cells. |
-Tongue adenosquamous carcinoma (Jiang et al., 2009) -56 yrs, male -Stage NA? -Poorly differentiated -Patient was not treated before biopsy |
|
|
How is ceramide produced? (3) |
1. De novo synthesis 2. Sphingomyelin hydrolysis 3. Salvage pathway – breakdown of complex sphingolipids to sphingosine, which is re-used to form ceramide
|
|
|
List the SMase genes and their corresponding proteins (5). |
1. SMPD1/acidic SMase (aSMase)/secretory SMase(sSMase) 2. SMPD2/nSMase1 3. SMPD3/nSMase 2 4. SMPD4/nSMase3 5. ENPP7/alkaline SMase (bSMase) |
|
|
What is palmitoylation? What are its functions (3)? |
Palmitoylation is the covalent attachment of fatty acids to cysteine, or less frequently to serine or threonine, residues of proteins. Its functions include: 1. Enhancement of protein hydrophobicity, which alters its affinity for cell membranes. 2. Subcellular trafficking of proteins among cell compartments. 3. Modulation of protein-protein interactions. |
|
|
Describe the functions and localization of SMPD1. |
Acidic and secretory SMase arise from the same gene but differ in their trafficking. aSMase is localized to lysosomes and the outer PM whereas sSMase is localized to the Golgi secretory pathway . Targeting depends on grade of mannosylation (sSMase is low in mannosylation). Deficiency is associated with Niemann-Pick disease. |
|
|
Describe the functions and localization of SMPD2/nSMase1. |
Localized to the ER; expressedin all cell types; mouse KO has no phenotype. |
|
|
Describe the characteristics (functions, localization, tissue distribution, mouse KO phenotype) of SMPD3. |
Expression is ubiquitous but highest in brain, bone and gut; activated by TNFα and contributes to TNF-induced apoptosis in culture; mouse KOs display developmental defects including dwarfism and delayed puberty (attributed to hypothalamic pituitary deficiency) and skeletal deformities; dependent on Mg2+ or Mn2+; optimal pH of ~7; 71 kD; localized to PM and subcellular membranes; characterized by 2 palmitoylation sites required for stability and localization. |
|
|
Describe the functions and localization of SMPD4. |
Localized to the ER and Golgi. Initially thought to be a sphingomyelinase due to sequence homology. More recent research suggests it functions as a lysophospholipase. |
|
|
T1 |
Tumor 2 cm or less in greatest dimension |
|
|
T2 |
Tumormore than 2 cm but not more than 4 cm in greatest dimension |
|
|
T3 |
Tumorgreater than 4 cm in greatest dimension |
|
|
T4a (oral cavity) |
Tumor invades through cortical bone into deep/extrinsic muscle of tongue (genioglossus, hyoglossus, palatoglossus, and styloglossus), maxillary sinus, or skin of face. |
|
|
T4b |
Tumor invades masticator space, pterygoid plates, or skull base, or encases internal carotid artery |
|
|
N1 |
Metastasis in a single ipsilateral (same side) lymph node, 3 cm or less in greatest diameter |
|
|
N2a |
Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension |
|
|
N2b |
Metastasis in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension |
|
|
N2c |
Metastasis in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension |
|
|
N3 |
Metastasis in a lymph node more than 6 cm ingreatest dimension |
|
|
Define adenosquamous carcinoma. |
A rare aggressive neoplasm originating from the surface epithelium and which is characterized by both SCC and true adenocarcinoma. |
|
|
Define verrucuous carcinoma. |
An exophytic, warty, slow growing variant of SCC with pushing margins. |
|
|
Define basaloid squamous cell carcinoma |
An aggressive, high-grade variant of SCC composed of both basaloid and squamous components. Also called adenoid cystic-like carcinoma.
|
|
|
Define papillary squamous cell carcinoma |
A distinct variant of SCC characterized by an exophytic, papillary growth and a favourable prognosis. Rarely recognized in the oral cavity and oropharynx other than as a component of a large SCC. |
|
|
Define spindle cell carcinoma |
A biphasic tumor composed of squamous cell carcinoma, either in-situ and/or invasive, and a malignant spindle cell component with a mesenchymal appearance but of epithelial origin. |
|
|
Define acantholytic squamous cell carcinoma. |
An uncommon histopathologic variant of SCC characterized by acantholysis (loss of intercellular connections such as desmosomes, resulting in loss of cohesion between keratinocytes) of the tumor cells, creating pseudolumina and false appearance of glandular differentiation. Lip is the most frequent oral site. |
|
|
Define carcinoma cuniculatum |
Rare variant of oral cancer in which tumor infiltrates deeply into bone. Oral tumors show proliferation of stratified squamous epithelium in broad processes with keratin cores and keratin filled crypts which seem to burrow into bone, but lack obvious cytological features of malignancy |
|
|
Define lymphoepithelial carcinoma |
A poorly differentiated SCC or undifferentiated carcinoma accompanied by a prominent reactive lymphoplasmacytic infiltrate (i.e., with characteristics of lymphocytes and plasma cells). |
|
|
How do aneuploidy and LOH relate to cancer progression? |
A strong positive correlation exists between degree of aneuploidy and progression. LOH on chromosomes 3p and 9p seem to be particularly important for predicting progression risk. |
|
|
Stage 0 |
Tis (carcinoma in-situ), N0, M0 |
|
|
Stage I |
T1, N0, M0 |
|
|
Stage II |
T2, N0, M0 |
|
|
Stage III (2) |
1. T1/T2, N1, M0 2. T3, N0/N1, M0 |
|
|
Stage IVA (2) |
1. T1/T2/T3, N2, M0 2. T4a, N0/N1/N2, M0 |
|
|
Stage IVB (2) |
1. Any T, N3, M0
2. T4b, Any N, M0 |
|
|
Stage IVC |
Any T, Any N, M1 |
|
|
Head and neck cancer includes malignancies of which body parts? (6) |
1.Oral cavity 2. Oropharynx 3. Hypopharynx 4. Larynx 5. Paranasal sinuses 6. Salivary glands |
|
|
EGFR belongs to what protein family? |
The ErbB family of transmembrane tyrosine kinases |
|
|
Name the ligands of EGFR (4). |
1. EGF 2. TGF-alpha 3. Amphiregulin 4. Beta-cellulin |
|
|
What major signaling pathways are activated by EGFR? (4) |
1. PI3K-AKT-mTOR 2. Ras-MAPK 3. PLC-gamma-PKC 4. JAK/STAT |
|
|
What is Cetuximab? |
Cetuximab is a chimeric mAB that targets the extracellular ligand-binding domain of EGFR, blocking downstream signaling; improves overall survival in locally advanced and recurrent/metastatic disease in combination with radiotherapy and platinum-based chemotherapy, respectively |
|
|
List possible mechanisms of Cetuximab resistance (3) |
1. Overexpressionof the tumor-specific EGFR deletion mutant EGFRvIII (which is constitutively active) 2. Epithelial-to-mesenchymal transition 3. Activation of redundant receptor tyrosine kinase signaling pathways |
|
|
Describe the composition of PI3K |
A heterodimer consisting of a regulatory subunit (p85) and a catalytic subunit (p110) |
|
|
List the steps in the PI3K-AKT-mTOR pathway (4) |
1. After activation by receptor tyrosine kinases, PI3K converts phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3) 2. PIP3 recruits pleckstrin homology (PH) domain-containing proteins such as AKT/protein kinase B to the plasma membrane 3. AKT is phosphorylated by phosphoinositide-dependent protein kinase 1 (PDK-1) and mammalian target of rapamycin complex 2 (mTORC2) 4. Phosphorylation activates AKT, which in turn phosphorylates proteins involved in anabolic cell survival processes |
|
|
What is the function of PTEN? |
Protein phosphatase and tensin homology (PTEN)is a well-known tumor suppressor which converts PIP3 to PIP2, thus blocking thePI3K pathway |
|
|
What are JAKs? |
Janus kinases (JAKs) belong to a family of non-receptor tyrosine kinases that are activated by cytokine binding to receptors |
|
|
How do JAK proteins work? |
Constitutively bound JAK molecules activate cytokine receptors by transphosphorylation; these activated cytokine receptors phosphorylate signal transducer and activator of transription (STAT) proteins which dimerize and translocate to the nucleus where they act as transcription factors |
|
|
2 scenarios resulting in tumor hypoxia. |
1. Aberrant tumor vasculature (acute hypoxia) 2. Diffusion-limitation due to high metabolic demands within tumors (chronic hypoxia) |
|
|
Define hypoxia |
A region of oxygen partial pressure <10 mm Hg, compared to 20-70 mm Hg in normal tissues |
|
|
How does the Warburg Effect change microenvironment pH? |
The Warburg Effect (aerobic glycolysis) resultsin microenvironment acidosis due to increased lactate efflux, which maycontribute to increased invasiveness and poor prognosis by activating proteasesand disrupting cell adhesion molecule function |
|
|
What is the significance of carbonic anhydrase (CA) IX? What is its function? |
Carbonic anhydrase (CA) IX, a transmembrane zinc metalloenzyme, is considered the most sensitive endogenous marker of hypoxia.It regulates intracellular pH by catalyzing the reversible reaction CO2 + H2O<-> HCO3 + H+ in the extracellular space. Bicarbonate ions are imported into the cytoplasm while the protons contribute to extracellular acidoosis |
|
|
List the two main signaling pathways through which ceramide promotes apoptosis. |
1. Mitochondrial pathway 2. Stress-acivated protein kinase (SAPK/p38-MAPK) pathway Note: Evidence suggests functional signaling through both pathways required for ceramide induced apoptosis |
|
|
Describe the mitochondrial pathway of ceramide-induced apoptosis |
1. Increased ceramide activates protein phosphatase 2A (PP2A) 2. PP2A dephosphorylates the apoptotic proteins Bak and Bax (resulting in their activation) and the anti-apoptotic protein Bcl-2 (resulting in its proteasomal degradation) |
|
|
List the ways in which ceramide can be metabolized (4) |
1. Deacylation to form sphingosine 2. Phosphorylation to form ceramide-1-phosphate (C1P) 3. Glycosylation to form glucosylceramide 4. Incorporation into sphingomyelin by sphingomyelin synthase |
|
|
How is sphingosine-1-phosphate formed from ceramide and how does it contribute to cancer? |
Ceramide may be metabolized to sphingosine, which is quickly phosphorylated to sphingosine-1-phosphate (S1P). S1P promotes cancer by inhibiting apoptosis, enhancing proliferation, transformation and angiogenesis, and contributing to inflammation |
|
|
What are the functions of ceramide transport protein (CERT)? How does it contribute to cancer? |
CERT transports newly formed ceramide from the ER to the Golgi. CERT is commonly upregulated in cancer and is thought to be linked to multidrug resistance |
|
|
What are the functions of glucosylcermide synthase (GS) and how does it contribute to cancer? |
GS glycosylates ceramide, therby reducing its concentration within the cell. Increased GS has been linked to multidrug resistance |
|
|
How is ceramide-1-phosphate (C1P) formed and how does it contribute to cancer? |
Ceramide can be phosphorylated to C1P by ceramide kinase. C1P promotes inflammation and increases cell survival by activating the PI3K-Akt pathway |
|
|
Use of exogenous ceramide and sphingolipid analogs is limited by these factors (3) |
1. Low solubility 2. Low cell permeability 3. Lack of specificity |
|
|
How are the limitations of exogenous ceramide treatment being overcome? |
1. Liposomal delivery - studies in mice show that incorporation of ceramide into synthetic liposomes is both safe and effective 2. Chemical modification - cationic water-soluble pyridinium ceramide analogs possess a positive charge which increases their solubility |
|
|
What are the functions of acid ceramidase and how does it contribute to cancer? |
Acid ceramidase catabolizes ceramide to sphingosine, which is quickly phosphorylated to sphingosine-1-phosphate. S1P promotes cancer in various ways (see other card) |
|
|
What is safingol? How is it relevant to cancer treatment? |
Safingol is an inhibitor of PKC and sphingosine kinase 1. It has been shown to reduce cell adhesion and induce apoptosis in oral SCC cells and is currently undergoing Phase II clinical trials |
|
|
Contrast methylation in vertebrates to that in plants and invertebrates |
-In vertebrates, cytosine methylation occurs throughout the entire genome -In plants and invertebrates, methylation is 'mosaic', meaning only specific genomic elements are targeted (repetitive elements and actively transcribed sequences are methylated) |
|
|
How does whole-genome methylation affect the genome over evolutionary time? |
CpG depletion through inefficient base-excision repair. C to T transition at CpG's is the most frequent mutation observed in human disease and between closely related mammals |
|
|
Describe the histone modifications and DNA methylation changes that occur at inactive CGI promoters |
-Inactive CGI promoters, in general, do not acquire DNA methylation. -They are often marked by H3K27 tri-methylation, making them more susceptible to DNA methylation during differentiation or disease states -May be marked with H3K4me independent of activity; this modification is throught maintain the hypomethylated state of CGIs by inhibiting de novo methyltransferases |
|
|
Describe how methylation changes at CG-poor regulatory regions when occupied by transcription factors |
-CG-poor regions are generally methylated -Binding of TFs to methylated CG-poor regions causes demethylation |
|
|
Name the proteins involved in establishing and maintaining DNA methylation (4) |
De novo methyltransferase: DNMT3A, DNMT3B, DNMT3L Maintenance methyltransferase: DNMT1 |
|
|
Describe 2 mechanisms by which DNA methylation may be lost |
1. Passively, through imperfect maintenance 2. Actively, catalyzed by ten-eleven translocation (TET) family members |
|
|
Describe active DNA demethylation by TET proteins |
1. TET proteins convert 5-methylcytosine to 5-hydroxymethylcytosine 2. further iterative oxidations result in 5-formylcytosine and 5-carboxylcytosine which are removed by thymine DNA-glycosylase (TDG) |
|
|
List the 2 protein domains known to recognize the CG dinucleotide based on its methylation state |
1. Methyl CpG-binding domain (MBD) - proteins with this domain recognize methylated CG dinucleotides 2. CXXC-domain - proteins containing this domain recognize unmethylated CGs and can target other proteins to unmethylated CGIs |
|
|
List common contaminants of EV preps (5) |
1. Lipoproteins 2. Microbes 3. Microsomes 4. DNA/Chromatin 5. Protein aggregates |
|
|
What miRs are known/thought to regulate the expression of SMPD3? |
1. miR-18b (Liu et al., 2016) 2. miR-632 (Liu et al., 2016) |
|
|
Where is SMPD3 located in the human genome? |
16q22.1 |
|
|
What are the functions of Rab27a and Rab27b in exosome biogenesis? |
Rab27a/b regulate the transport of MVBs to the plasma membrane. |
|
|
How are early and late stage HNSCCs treated? |
Early stage tumors are treated with either surgery or radiation. Late stage tumors are treated with surgery followed by postoperative radiation. Chemotherapy may also be used in combination with radiation some cases. |
|
|
List the most commonly used chemotherapy drugs for HNSCC and describe how they work. |
Cisplatin - Platinum-based drug which binds DNA causing it to crosslink. This then trigger DNA repair and/or apoptosis. Cetuximab - chimeric (mouse/human) monoclonal antibody targeting EGFR. Only works in patients with wt Kras as Kras is downstream of EGFR. 5-fluorouracil - Mainly works by inhibiting Thymidylate synthase, which is required for the synthesis of thymine. Lack of thymine causes rapidly dividing cells to undergo cell death Carboplatin - Like cisplatin, Carboplatin contains platinum and works by interfering with DNA replication. However there are two theories on how it works at the molecular level which are too detailed to explain here Paclitaxel - Stabilizes microtubules and protects them from disassembly, thereby blocking chromosomes from achieving a metaphase spindle configuration thereby blocking mitosis and triggering apoptosis |
|
|
Name and describe the main methods of radiation given to HNSCC patients (2) |
1. External beam therapy (EBT) - A beam of high-energy x-rays or protons generated outside the patient and targeted to the tumor 2. Intensity-modulated radiation therapy (IMRT) - utilizes computer-controlled x-ray accelerators to deliver precise radiation doses to a tumor in 3D while minimizing radiation exposure to healthy cells |
|
|
Which strain of HPV is most commonly associated with OPC? Describe p53 and prognosis in these patients. |
HPV16. These tumors tend to have wild-type TP53 and good prognosis |
|
|
Describe the common molecular alterations which occur during progression of a cancer field in HNSCCs. |
-LOH at chromosomes 3p, 9p, and 17p -TP53 mutation -Alteration of p16^INK4a (p16; encoded by the gene CDKN2A) |
|
|
Do HPV +'ve oropharyngeal cancers undergo field cancerization? Provide evidence for your claim. |
Unlikely. Progressing field cancerization is characterized by LOH at chromosomes 9p, 3p, and 17p as well as TP53 mutation and alteration of p16INK4a. However, HPV +'ve OPSCCs tend to lack these molecular alterations, typically having wild-type TP53 and normal p16 expression. |
|
|
Somatic TP53 mutations are found in ____% of HNSCCs. |
60 - 80% |
|
|
How do alterations of p16 and cyclin D1 contribute to HPV -'ve HNSCC? |
p16, encoded by the CDKN2A gene, is a cell cycle regulator and tumor suppressor commonly lost in HNSCCs by methylation or mutation in combination with chromosome loss or homozygous deletion. Cylcin D1, encoded by the CCND1 gene, is also a cell cycle regulator and is found to be amplified or gained in >80% of HPV -'ve HNSCCs |
|
|
List the hallmarks (6) and emerging hallmarks (2) of cancer as defined by Hanahan and Weinberg. |
1. Sustained proliferative capability 2. Evading growth suppressors 3. Activating invasion and metastasis 4. Enabling replicative immortality 5. Inducing angiogenesis 6. Resisting cell death
Emerging: 1. Avoiding immune destruction 2. Reprogramming energy metabolism |
|
|
How do cisplatin and cetuximab affect tumor response to radiation? |
Both cisplatin and cetuximab are considered radiosensitizers and are often used in combination with radiation to treat locally advanced/metastatic HNSCC |
|
|
What is the total dose of radiation typically given to HPV +'ve OPSCC patients. |
66 - 74 Gy in combination with cisplatin, though investigations into reduced-intensity radiotherapy (max dose 60 Gy) with or without cisplatin are ongoing |
|
|
How is PET-CT imaging being applied to manage HNSCC? |
PET-CT imaging allows detection of residual disease after chemoradiation therapy. Patients with no residual disease may avoid having additional surgery. |
|
|
Name the two anti-PD1 antibodies approved in 2016 for the treatment of HNSCC patients with platinum-refractory recurrent and/or metastatic disease. |
1. Pembrolizumab 2. Nivolumab |
|
|
Name the 2 enabling characteristics required for acquisition of the hallmarks of cancer as defined by Hanahan and Weinberg |
1. Genome instability 2. Inflammation |
|
|
How do cancer cells acquire the capability to sustain proliferative signaling? (6) |
1. Production of a growth factor ligands and response to those ligands and response via expression of cognate receptors (autocrine) 2. Stimulation of tumor-associated stromal cells, which respond by supplying growth factors to cancer cells 3. Elevated expression of receptors, which makes cell hypersensitive to ligands 4. Structural alteration of receptors, making them ligand-independent/constitutively active 5. Constitutive activation of downstream signaling pathways 6. Defects in negative feedback mechanisms |
|
|
Describe the steps of Bax-Bak activated apoptosis. |
1. Bax and Bak are normally inhibited by anti-apoptotic proteins such as Bcl-2 2. Once activated, Bax and Bak disrupt the integrity of the outer mitochondrial membrane 3. Disrupted mitochondria results in release of proapoptotic proteins including cytochrome c 4. Cytochrome c results in the activation of various caspases 5. These proteases result in disassembly of the cell |
|
|
Define autophagy. |
The breakdown of cellular components/organelles and recycling of the resulting catabolites for biosynthesis and energy metabolism |
|
|
Function of Beclin-1 |
Beclin-1 is thought to be required for the induction of autophagy |
|
|
What is the significance of the BH3 domain? |
The BH3 domain is a protein-protein interaction domain. A subfamily of related proteins sharing a single BH3 domain are involved in triggering apoptosis by interfering with antiapoptotic Bcl-2 or by stimulating proapoptotic family members |
|
|
p53 triggers apoptosis through upregulation of these 2 BH3-domain containing proteins |
1. Noxa 2. Puma |
|
|
Necrosis |
-Form of cell death -Cells bloat and explode, releasing their contents -Increasing evidence for genetic control -Results in release of factors that attract inflammatory cells. Such cells promote tumorgenesis by releasing growth factors |
|
|
Cell senescence |
-A typically irreversible nonproliferative but viable state -Possibly induced by high levels of oncogenic signaling and/or subcritical shortening of telomeres |
|
|
Cell crisis |
Cell able to overcome senescence and resume proliferation often enter crisis, resulting in cell death |
|
|
Immortalization |
The process by which cells emerge from a population in crisis but rather than undergoing cell death instead show unlimited replicative potential |
|
|
Telomere |
The ends of chromosomes, composed of multiple tandem hexanucleotide repeats |
|
|
What are the functions of telomeres? |
-Telomeres protect the ends of chromosomal DNA from end-to-end fusion events -Such events would generate unstable dicentric chromosomes -Resolution of such chromosomes results in a scrambling of karyotype that threatens cell viability -Length of telomeric DNA dictates how many successive cell generations its progeny can pass through |
|
|
How might transient telomere deficiency enhance human tumorigenesis? |
It has been hypothesized that, in the absence of p53, cells are able to survive initial telomere erosion and resulting chromosomal breakage-fusion-bridge cycles. Such cycles increase genomic instability, thus accelerating mutations in oncogenes/tumor suppressors |
|
|
Define vasculogenesis and angiogenesis |
Vasculogenesis - The birth of new endothelial cells and their assembly into tubes Angiogenesis - Sprouting of new vessels from existing ones |
|
|
What is the best known inducer and inhibitor of angiogenesis? |
Inducer - Vascular endothelial growth factor (VEGF) Inhibitor - Thrombospondin-1 (TSP-1) |
|
|
List the characteristics of abnormal tumor vasculature (7). |
1. Precocious capillary sprouting 2. Convoluted and excessive vessel branching 3. Distorted and enlarged vessles 4. Erratic blood flow 5. Microhemorrhaging 6. Leakiness 7. Abnormal levels of EC proliferation and apoptosis |
|
|
List the steps in the invasion-metastasis cascade (6). |
1. Local invasion 2. Intravasation into nearby blood/lymph vessels 3. Transfer of cancer cells through the lymph/hematogenous systems 4. Extravasation into parenchyma of distant tissues 5. Formation of small nodules of cancer cells (micrometastases) 6. Colonization, i.e., growth into macroscopic tumors |
|
|
List and describe the main modes of invasion. |
1. Mesenchymal - Individual cells attain a mesenchymal phenotype via complete or partial EMT 2. Collective invasion - Nodules of cancer cells advance en masse into adjacent tissues 3. Ameboid - Individual cancer cells show morphological plasticity, enabling them to slither through the ECM rather than clearing a path as occurs in the above modes |
|
|
What invasive mode is characteristic of SCCs? |
Collective invasion |
|
|
How does inflammation contribute to cancer hallmark capabilities? |
Inflammatory cells provide multiple bioactive molecules to cells of the tumor microenvironment. This may include growth factors for sustained proliferation, survival factors that limit cell death, ECM modifying enzymes that facilitate angiogenesis/invasion/metastasis, and inductive signals leading to EMT and other programs. In addition, inflammatory cells can release chemicals such as ROS that are actively mutagenic |
|
|
Cancer cells are known to favor glycolysis even under aerobic conditions. Why might this be so? |
One hypothesis is that increased glycolysis allows the diversion of glycolytic intermediates into various biosynthetic pathways, which in turn facilitates the biosynthesis of macromolecules and organelles required to assemble new cells |
|
|
What are pericytes? What are their functions? |
A specialized mesenchymal cell type related to smooth muscle cells with finger-like projections that wrap around the endothelial tubing of blood vessels. Provide mechanical and physiology support (via paracrine signaling) to endothelial cells. |
|
|
What are the 2 subtypes of cancer-associated fibroblast? |
1. Cells derived from recruited fibroblasts 2. Cells derived from recruited myofibroblasts, which express alpha smooth muscle actin |
|
|
List 4 ways that tumor-associated stromal cells may be supplied to growing tumors. |
1. Proliferation of preexisting stromal cells. 2. Differentiation in situ of local stem/progenitor cells 3. Differentiation in situ of stem/progenitor cells originating in neighboring normal tissue 4. Recruitment of bone marrow-derived stem/progenitor cells |
|
|
In what year was TORS first used for transoral cancer dissection? |
2004 |
|
|
List the components of the TORS system (4) |
1. Surgeon console(s) 2. Patient cart with robotic arms 3. Robotic instruments 4. Mobile tower cart with HD monitor system |
|
|
According to the FDA, which patients are candidates for TORS? |
TORS is restricted to those patients with benign and cancerous tumors in otolaryngology procedures classified as T1 and T2 (early and midstage) and for benign tongue base resection procedures |
|
|
Briefly describe the release of exosomes and microvesicles. |
1. Exosomes are released on fusion of the MVB with the plasma membrane. 2. Microvesicles are released by direct budding of the plasma membrane |
|
|
Name and describe the commonly used methods of exosome isolation/purification (4) |
Differential ultracentrifugation - Debris and microvesicles are pelleted and discarded. Exosomes are then collected at 110,000g Density gradient centrifugation - sometimes used in addition to DU to remove contaminants such as protein aggregates. Exosomes are collected at a density of 1.08 - 1.22 g/ml Immunoaffinity isolation - Use of antibody-coated magnetic beads which selects only those exosomes with a specific protein target on their surface. However, true exosome-specific markers don't yet exist Polymer-based methods (i.e., ExoQuick, others) |
|
|
Compare and contrast the protein content of microvesicles and exosomes. |
-There is overlap between the two -Membrane proteins of microvesicles tend to be more similar to that of the parental cell compared to exosomes -Exosomes tend to be enriched with endosomal proteins and those involved in MVB formation, as well as from the cytosol and PM, but lack proteins from the nucleus, ER, Golgi, and mitochondia. |
|
|
ISEV claims 3 protein markers are required to claim that an isolated population of EVs are exosomes. What are they? |
1. Presence of transmembrane or lipid-bound extracellular proteins (CD9, CD63, CD81, cell adhesion molecules, growth factor receptors, heterotrimeric G proteins, integrins, or MFGE8) 2. Presence of cytosolic proteins (TSG101, annexins, RAB GTPases, syntenin) 3. Absence of intracellular proteins (from Golgi, ER, mitochondria, nucleus or Argonaute/RISC) |
|
|
List the mechanisms by which miRNAs may be sorted into exosomes. |
1. nSMase2-dependent pathway 2. Sumoylated heterogeneous nuclear ribonucleoprotein (hnRNP)-dependent pathway 3. MiRNA 3'-end sequence-dependent pathway 4. RISC-related pathway(s) |
|
|
EVs are known to exist in which bodily fluids? |
1. Blood 2. Urine 3. Saliva 4. Breast milk 5. Amniotic fluid 6. Ascites 7. Cerebrospinal fluid |
|
|
Which fluid - plasma or serum - does ISEV suggest EVs be collected from for use as biomarkers and why? |
Plasma, because platelets release EVs in serum during clot formation which may account for >50% of EVs in serum |
|
|
Define clinical biomarker |
Any cellular, biochemical, molecular, or genetic alterations by which a normal, abnormal, or simlpe biological process can be recognized or monitored (Drucker and Krapfenbauer, 2003; Rahim et al., 2015) |
|
|
List the criteria a biomarker must fulfill to be considered for clinical use (5) |
1. Specific to a condition 2. Sensitive 3. Accessible in a practical manner 4. Easily tested for 5. Convey clinically actionable information |
|
|
When were microRNAs discovered? When were they found to be present in humans? |
1. 1993 (Lee et al., 1993) 2. 2000 (van Rooij, 2011) |
|
|
What features of microRNAs make them attractive biomarkers? |
1. Deregulated in disease 2. Can be collected non-invasively (blood, urine, brushings, etc.) 3. Highly stable, even under harsh conditions that degrade other molecules |
|
|
Why is miR-21 considered an unreliable biomarker in blood-based studies? |
MiR-21 was found to be the most highly expressed miR in vascular endothelial cells (Greco and Martelli, 2014). ECs have been proposed to be the major contributors to miRs in the peripheral blood |
|
|
What are Tzanck cells? |
Degenerated epithelial cells formed as a result of acantholysis from the spinous layer of the skin. Seen in various infectious and autoimmune disorders. |
|
|
HPV16 is responsible for approximately what percentage of HPV +'ve OPSCCs? |
90-95% (Chaturvedi et al., 2011) |
|
|
Approximately what percent of OPSCCs are caused by HPV infection? |
70% (Chaturvedi et al., 2011) |
|
|
What cell type is infected by HPV to cause OPSCC? |
Basal keratinocytes, i.e., keratinocytes of the stratum basale |
|
|
The oropharynx has what type of epithelium? |
Non-keratinized stratified squamous epithelium |
|
|
What is the relevance of p16 to HPV +'ve OPSCC? |
The viral protein E6 binds and inhibits the retinoblastoma protein. An increase in keratinocyte p16 expression occurs as an attempt to control the unchecked proliferation resulting from retinoblastoma inhibition. Thus, p16 is used as a marker for integration of viral DNA into the host genome. |
|
|
Why are men more likely than women to have an oral HPV infection? |
Possibly due to higher viral load in female genital mucosa vs male genital mucosa/skin |
|
|
How do the presence of HPV16 and HPV16 antibodies effect OPSCC risk? |
1. Serologically HPV-positive patients have 14x risk vs HPV16-negative patients (Mork et al., 2001) 2. Patients positive for E6/E7 antibodies have 73x risk vs patients negative for antibodies (Smith et al., 2007) |
|
|
Do smoking and immune status alter the risk of HPV +'ve OPSCC? |
Yes. Smoking reduces the body's ability to clear the virus. Reduced immune ability, such as in patients with HIV, also decreases viral clearance. |
|
|
Compare the following features of HPV +'ve vs -'ve OPSCC: 1. Overall recurrence rate. 2. Rate of distant metasteses. 3. Timing of distant metasteses. 4. Rate of secondary primaries. |
1. Overall recurrence rate is lower in HPV +'ve patients 2. Distant metasteses are more common in HPV -'ve patients 3. For HPV -'ve OPSCC, distant metasteses typically develop in <2 yrs from initial treatment; for HPV +'ve OPSCC, may develop more than 2 yrs after initial treatment 4. HPV +'ve patients less likely to develop secondary primaries |

