![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
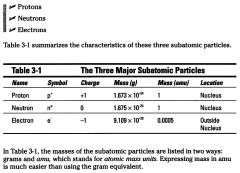
Name the atoms three Major Subatomic particles:
|
Protons
Neutrons Electrons |
|
|
|
What are Ions?
|
Atoms that gain a charge, either positive or negative, are called ions.
|
|
|
|
In 1911, Ernest Rutherford discovered....
|
In 1911, Ernest Rutherford discovered that atoms have a nucleus - a centercontaining
protons. Scientists later discovered that the nucleus also houses the neutron. |
|
|
|
Mass Number:
|
The sum of the number of protons plus the number of neutrons in an atom is called the Mass Number. The number of protons in a particular atom is given a special name, the atomic number.
|
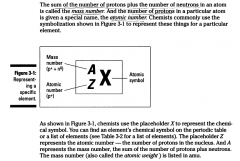
Chemists commonly use the symbolization shown in Figure 3-1 to represent these things for a particular element.
|
|
|
Atomic Number:
|
The number of protons in a particular atom is given a special name, the atomic number.
|
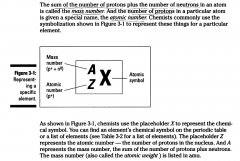
Chemists commonly use the symbolization shown in Figure 3-1 to represent these things for a particular element.
|
|
|
How do you find the number of neutrons in an atom?
|
Subtract the atomic number from the mass number.
|
You know that uranium has an atomic number of 92 (number of protons) and mass number of 238 (protons plus neutrons). So if you want to know the number of neutrons in uranium, all you have to do is subtract the atomic number (92 protons) from the mass number (238 protons plus neutrons). The resulting number shows that uranium has 146 neutrons.
But how many electrons does uranium have? Because the atom is neutral (it has no electrical charge), there must be equal numbers of positive and negative charges inside it, or equal numbers of protons and electrons. So there are 92 electrons in each uranium atom. |
|
|
The principle Quantum number n
|
The principal quantum number n describes the average distance of the orbital from the nucleus - and the energy of the electron in an atom. It's really about the same as Bohr's energy-level numbers. It can have positive integer (whole number) values: 1, 2, 3, 4, and so on. The larger the value of n, the higher the energy and the larger the orbital. Chemists sometimes call the
orbitals electron shells. |
|
|
|
The Angular momentum Quantum number I
|
The angular momentum quantum number I describes the shape of the orbital, and the shape is limited by the principal quantum number n: The angular momentum quantum number I can have positive integer values from 0 to n-1. For example, if the n value is 3, three values are allowed for I: 0, 1, and 2. The value of I defines the shape of the orbital, and the value of n defines the size.
Orbitals that have the same value of n but different values of I are called subshells. These subshells are given different letters to help chemists distinguish them from each other. Table 3-4 shows the letters corresponding to the different values of I. |
|
|
|
The magnetic Quantum number m1
|
The magnetic quantum number ml describes how the various orbitals are oriented in space. The value of mt depends on the value of t. The values allowed are integers from -t to ° to +t. For example, if the value of t = 1 (p orbital- see Table 3-4), you can write three values for mt: -1, 0, and +1. This means that there are three different p subs hells for a particular orbital. The subshells have the same energy but different orientations in space.
Figure 3-4 (b) shows how the p orbitals are oriented in space. Notice that the three p orbitals correspond to mt values of -1,0, and +1, oriented along the x, y, and z axes. |
|
|
|
What is the Aufbau Principle?
|
Aufbau Principle, a method for remembering the order
in which orbitals fill the vacant energy levels. |
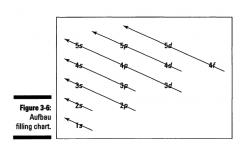
In using the energy level diagram, remember two things: IyI Electrons fill the lowest vacant energy levels first.
When there's more than one subshell at a particular energy level, such as at the 3p or 4d levels (see Figure 3-5), only one electron fills each subshell until each subshell has one electron. Then electrons start pairing up in each subshell. This rule is named Hund's Rule. |
|
|
Valence Electrons:
|
The electrons in the outermost energy level are commonly called valence electrons.
|
|
|
|
Isotopes:
|
But then again, I'm a nerd. The atoms in a particular element have an identical number of protons and electrons but can have varying numbers of neutrons. If they have different numbers of neutrons, then the atoms are called isotopes.
|
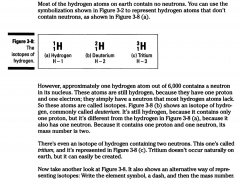
|
|
|
Ions and Cations & Anions
|
Because an atom itself is neutral, throughout this book I say that the number of protons and electrons in atoms are equal. But there are cases in which an atom can acquire an electrical charge. For example, in the compound sodium chloride - table salt - the sodium atom has a positive charge and the chlorine atom has a negative charge. Atoms (or groups of atoms) in which there are unequal numbers of protons and electrons are called ions.
|
The neutral sodium atom has 11 protons and 11 electrons, which means it has 11 positive charges and 11 negative charges. Overall, the sodium atom is neutral, and it's represented like this: Na. But the sodium ion contains one more positive charge than negative charge, so it's represented like this: Na+ (the + represents its net positive electrical charge). This unequal number of negative and positive charges can occur in one of two ways: An atom can gain a proton (a positive charge) or lose an electron (a negative charge). So which process is more likely to occur? Well, a rough guideline says that it's easy to gain or lose electrons but very difficult to gain or lose protons.
So atoms become ions by gaining or losing electrons. And ions that have a positive charge are called cations. The progression goes like this: The Na+ ion is formed from the loss of one electron. Because it lost an electron, it has more protons than electrons, or more positive charges than negative charges, which means it's now called the Na+ cation. Likewise, The Mg2+ cation is formed when the neutral magnesium atom loses two electrons. Now consider the chlorine atom in sodium chloride. The neutral chlorine atom has acquired a negative charge by gaining an electron. Because it has unequal numbers of protons and electrons, it's now an ion, represented like this: Cl". And because ions that have a negative charge are called anions, it's now called the Cl" anion. (You can get the full scoop on ions, cations, and anions in Chapter 6, if you're interested. This here's just a teaser.) |
|
|
Isoelectronic:
|
The electron configuration of the chloride ion (Cl) is:
IS2 2s2 2p6 3s2 3p6. This is the same electron configuration as the neutral Argon atom. If two chemical species have the same electron configuration, they're said to be isoelectronic. Figuring out chemistry requires learning a whole new language, eh? |
|
|
|
Monoatomic and Polyatomic Atoms
|
This section has been discussing monoatomic (one atom) ions. But
polyatomic (many atom) ions do exist. The ammonium ion, Mit" is a polyatomic ion, or, specifically, a polyatomic cation. The nitrate ion, N03·, is also a polyatomic ion, or, specifically, a polyatomic anion. |
|
|
|
Electrolyte:
|
Ions are commonly found in a class of compounds called salts, or ionic
solids. Salts, when melted or dissolved in water, yield solutions that conduct electricity. A substance that conducts electricity when melted or dissolved in water is called an electrolyte. Table salt - sodium chloride - is a good example. On the other hand, when table sugar (sucrose) is dissolved in water, it becomes a solution that doesn't conduct electricity. So sucrose is a nonelectrolyte. Whether a substance is an electrolyte or a nonelectrolyte gives clues to the type of bonding in the compound. If the substance is an electrolyte, the compound is probably ionically bonded (see Chapter 6). If it's a nonelectrolyte, it's probably covalently bonded (see Chapter 7). |
|

