![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
|
What mechanism do cells use for determining gene expression? |
Methylation of cytosine residues. |
|
|
In what ways does methylation get passed onto future generations? |
1. Imprinting 2. Chromatin structures propagated to daughter cells 3. Cytosine by a methyl transferase which is faithfully inherited |
|
|
What are CG dinucleotides? |
Are a type of dinucleotide motif which occur infrequently in vertebrate genomes but often cluster together to form CG islands |
|
|
How many CG islands are there in the human genome? |
20,000 |
|
|
How can you tell which CG islands are repressed or expressed? |
1. Methylated = Repressed 2. Unmethylated = Expressed |
|
|
What is the role of activator proteins? What does a lack of them result in? |
Activator proteins block DNA methylation Loss of activator = methylation and gene repression by further condensed packing of chromatin. |
|
|
How does methylation prevent gene expression? (in detail) |
1. Reciprocal interactions between DNA and histones: i) Modification of the H3 histone to be methylated - chromatin stabilisation ii) Methylation allows histone deacetylases (HDACs) to bind such as Sin3 which further stabilises the chromatin iii) H1 histones now preferentially bind to the methlated DNA - forming higher order chromatin structures and thus preventing gene expression. |
|
|
What is the activity of the beta globin gene related to? |
Activity of Beta globin is inversely correlated with the amount of methylation of their promoters. |
|
|
How does globin gene expression change throughout development>? |
In development, embryonic / fetal globin is expressed and adult chains are repressed. This changes over development and embryonic globin promoters become methylated - thus inactivating them |
|
|
What are the roles of DNA insulators? |
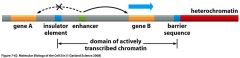
Insulators prevent inappropriate activation of methylated promoters. In addition the flanking insulator prevents inappropriate inactivation by methyltransferases of the gene which it is controlling |
|
|
Why do Barr bodies form? |
Only one X chromosome is required in a female XX cell so one is inactivated - tightly packaged and methylated. This is a form of dosage compensation - |
|
|
Describe the formation of a Barr body. |
Process begins with the transcription of XIST - x inactivation specific transcript from the XIC - X inactivation centre locus. The process of selection of which XIST locus is activated appears to be random. This results in widespread condensation of the X chromosome. |
|
|
What is the hallmark example of X chromosome inactivation? |
In tortoise shell cats where hair colour is x linked. |
|
|
Describe how cancer and gene expression are intrinsically linked. |
1. If a tumour suppressor gene is inappropriately inactivated by DNA methylation 2. If oncogenes are inappropriately demethylated and thus inactivated. |
|
|
How do tumours hijack the methylation system of DNA? |
Tumour cells commonly exhibit widespread DNA methyltransferase activity resulting in widespread loss of methylation and areas of DNA hypermethylation - Many areas of inappropriately activated and inactivated gene areas. |
|
|
How can the clonal origin of cancer be tracked? |
X inactivation is inherited in tumour cells as well and so can be used to track the founder cell. |
|
|
What kind of changes of DNA methylation occur over development? |
1. Highly methylated zygote DNA 2. Early development = mass demethylation 3. After implantation - de novo methylation occurs in all somatic cells 4. Germ cell DNA methylation occurs at later stages of gamete formation |
|
|
What is a special case of epigenetic function relating to control of gene function? |
Genomic imprinting |
|
|
Describe genomic imprinting. |
A set of 100-200 genes in placental animals which are hypermethylated or not methylated depending on which parent the allele was inherited from. This leads to monoallelic expansion in cells where the non imprinted version of the locus is expressed. |
|
|
What happens to imprinted genes in germ line formation? |
In the germ line formation the imprints are first erased and then reimposed according to the sec of the animal. |
|
|
Insulators are especially vital in imprinting - specifically, what do they do? |
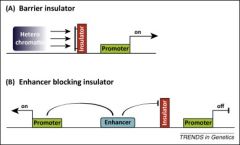
1. Prevent the spread of heterochromatin 2. DIrectionally block the action of enhancers. |
|
|
Describe the pattern of imprinting of the mouse IGF2 gene. |
1. In the maternal genome - the CTCF gene binds to an insulator locus element preventing the activation of IGF2 2. In the paternal genome - The CTCF gene is expressed however the insulator site is imprinted (heavily methylated) preventing CTCF binding and thus allowing transcriptional activation of IGF2 |
|
|
What are the common genes which are imprinted? Where can this be observed? |
Commonly, growth factor genes are repressed in the maternal gene line and expressed in the paternal gene line. In ligers - a female tiger and male lion cross the methylation patterns are reversed across the two big cat species. The females have growth factor initiation in tigers and in lions the males have growth factor stimulation. This leads to the Liger having double the amount of usual growth factors - making it massive. |
|
|
How is imprinting related to cancer? Give an example. |
Some cancers thought to develop in part due to loss of imprinting from one allele - relaxation of monoallelic expression. E.g Insulin like growth factor (IGF2) which causes Beta cell tumourigenesis, bladder and lung cancer. |
|
|
Describe the genetic causes of Prader-Willi and Angelman syndromes. |
Region in the long arm of Chromosome 15 q arm contains the genes affecting these syndromes. The Prader Wili genes - active in father - inactive in mother The Angelman syndrome genes - UBE3A are usually inactivated in the father and active in the mother. If the maternal 15q site is deleted leaving only expression of the prader wili genes and an imprinted UBE3A locus this results in angelman syndrome If the paternal 15q site is deleted leaving only maternal expression of the UBE3A genes and no prader wili genes. This results in Prader wili syndrome |

