![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back
|
Atomic number Z |
Number of protons |
|
|
Mass Number A |
Number of protons + neutrons |
|
|
Alpha decay |
4 alpha 2 Subtract 4 from top and 2 from bottom |
|
|
Beta- decay |
0 beta -1 So add +1 to proton number |
|
|
Beta + decay |
0 beta +1 So subtract 1 from proton |
|
|
Electron capture |
0 e- -1 Subtract 1 from protons |
|
|
Gamma decay |
Stays the same |
|
|
Electron configuration of negative and positive ions which way to move? |
Neg right positive left |
|
|
Atomic radius increases in which direction |

|
|
|
Ionization increases in which direction? |

|
|
|
Electron affinity becomes more negative in which direction? |

|
|
|
Electronegativity increases in which direction? |

|
|
|
Acidity increases in which direction? |
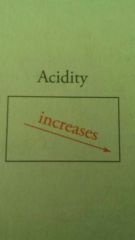
|

