![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
|
Equilibrium
|
Point in a reversible reaction where energy is minimized and entropy is maximized, concentration of products and reactants remain constant since the forward rate equals the rate of the reverse reaction (dynamic)
|
|
|
Law of Mass Action
|
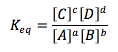
Only gases and aqueous species (no pure solids or liquids) |
|
|
Reaction Quotient
|
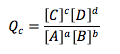
Used to compare the current concentration to the known Keq
|
|
|
Comparison of Q to Keq
|
Q < Keq: the reaction proceeds in the forward direction, ΔG < 0
Q = Keq: the reaction is in dynamic equilibrium, ΔG = 0 Q > Keq: the reaction proceeds in the reverse direction, ΔG > 0 |
|
|
Properties of the Law of Mass Action
|
Equilibrium constant is temperature dependent
The larger Keq the further to the right the equilibrium position If forward reaction is Keq reverse reaction is 1/Keq |
|
|
Le Chatelier Principle
|
When a chemical system experiences a stress, it will react so as to restore equilibrium
|
|
|
Shift Reaction to Right
|
Increase reactants, decrease products
Increasing temp of endothermic reaction, decrease temp of exothermic reaction |
|
|
Shift Reaction to the Left
|
Decrease reactants, increase products
Decrease temp of endothermic reaction, increase temp of exothermic reaction |
|
|
Effect of Changing Pressure on Reaction
|
Increasing pressure on gaseous system will shift reaction toward the side with fewer moles of gas
Decreasing pressure will shift towards side with more moles of gas |
|
|
Kinetic Product
|
Higher in free energy than thermodynamic products
Form at lower temperatures “Fast products” because they can form more quickly under such conditions |
|
|
Thermodynamic Product
|
Lower in free energy than kinetic products (more stable)
Proceed more slowly but more spontaneous |

