![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Boyle's Law
|
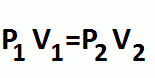
Temperature and number of moles are constant
Pressure and Volume are inversely related |

|
|
|
Charle's Law
|
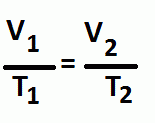
Pressure and number of moles are constant
Volume and Temperature are directly proportional |

|
|
|
Gay-Lussac's
|
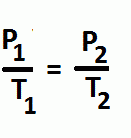
Volume and the number of moles are constant
Temperature and Pressure are directly proportional |

|
|
|
Combined Gas Law
|
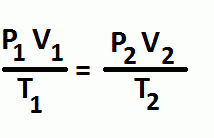
The number of moles is constant
|

|
|
|
What does STP stand for?
|
Standard Temperature and Pressure
|
|
|
|
What is STP?
|
Standard Temp.= 273K
Standard Pressure= 1 atm |
|
|
|
What is the Standard Molar Volume?
|
at STP, 1 mole of any gas has a volume of 22.4L
|
|
|
|
Avogadro's Law
|
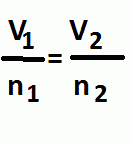
Pressure and Temperature are constant
Volume and number of moles are directly proportional |
|
|
|
Ideal Gas Law
|
PV=nRT
where R is [always] 0.0821 |
|

