![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
What is the pKa of the following hydrogens?
Alkane (sp3) Alkene (sp2) Alkyne (sp) |
Alkane ~55 (usually greater than)
Alkene 42 Alkyne ~25 |
|
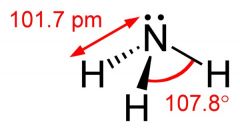
What is this and what is its pKa?
|
Ammonia, pKa 35
|
|

What is this and what is its pKa?
|
Alcohol, pKa 15-19 depending on the structure of R
|
|
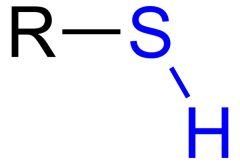
What is this and what is its pKa?
|
Thiol, pKa 10-12, depending on the structure of R.
|
|
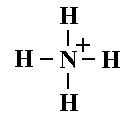
What is this and what is its pKa?
|
Ammonium ion, pKa 9.25
|
|
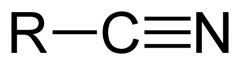
What is this and what is its pKa?
|
Hydrocyanic acid, pKa 9.40
|
|
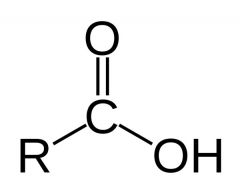
What is this and what is its pKa?
|
Carboxylic acid, pKa 1-5, depending on structure of R. Trifluoroacetic acid (HOOCCF3) has a pKa of less than 1 (about 0.5)
|
|
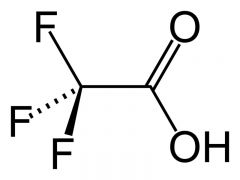
What is this and what is its pKa?
|
Trifluoroacetic acid, pKa ~0.50
|
|
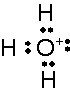
What is this and what is its pKa?
|
Hydronium ion, pKa, -1.7
|
|
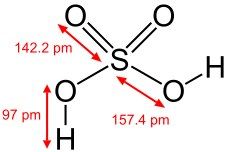
What is this and what is its pKa?
|
Sulfuric acid, pKa -3
|
|
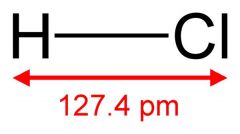
What is this and what is its pKa?
|
Hydrochloric acid, pKa -6 to -7
|
|
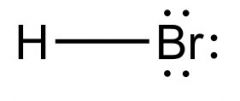
What is this and what is its pKa?
|
Hydrobromic acid, pKa -8 to -9.5
|
|

What is this and what is its pKa?
|
Hydroiodic acid, pKa -9.5 to -10
|
|
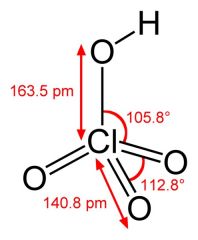
What is this and what is its pKa?
|
Perchloric acid, pKa -10 (actual not known)
|
|
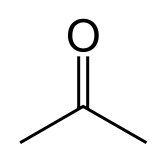
What is this and what is its pKa?
|
Ketone, pKa ~20
|
|

What is this and what is its pKa?
|
Allyl, pKa ~43
|
|
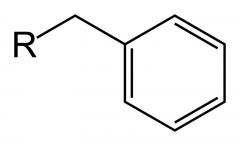
What is this? If R was a hydrogen, what is its pKa value?
|
A benzyl group, pKa 41
|
|
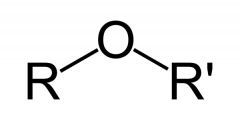
Name the functional group.
|
Ether
|
|
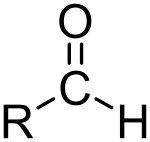
What is this and what is its pKa?
|
Aldehyde, pKa ~17
|
|
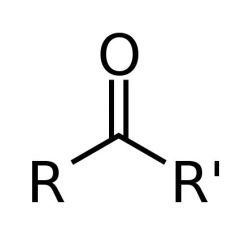
What is this and what is its pKa value?
|
Ketone, pKa ~20
|
|
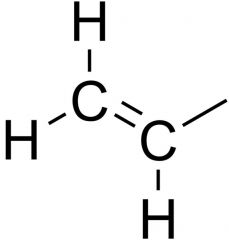
Name the functional group
|
Allyl group
|
|
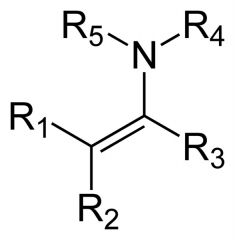
Name the compound
|
Enamine, this is the Nitrogen analog of an enol.
|
|
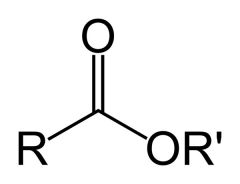
What is this and what is its pKa value?
|
Ester, pKa ~25
|
|
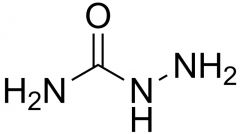
Name the compound.
|
Semicarbazide.
|
|
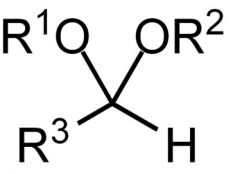
Name the functional group.
|
Acetal
|
|

Name the functional group.
|
Ketal
|
|
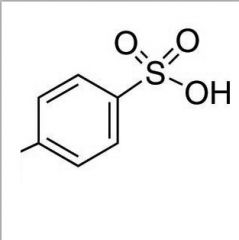
What is this and what is its pKa?
|
TsOH (p-toluenesulfonic acid), pKa ~ -1
|
|
|
What are the 4 categories of Carboxylic Acid reactions?
|
1) Reactions at the carbonyl group
2) Reactions at the carboxylate group 3) Loss of the carboxy group as CO2 (decarboxylation) 4) Reactions involving the α-carbon |
|
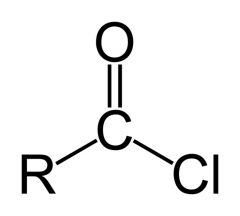
Name the functional group.
|
Acyl chloride (acid chloride).
|
|
|
What is a lactone?
|
A cyclic ester.
|
|
|
What's the difference between an amine and an amide?
|
An amide has a carbonyl group, an amine does not.
|
|
|
What is the pKa of a protonated amine?
|
~10.6
|
|
|
What is this and what is its pKa value?
|
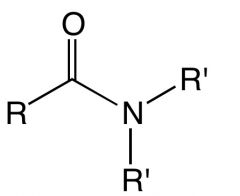
Amide
pKa ~15 |
|
|
What is this compound? How is it made?
|
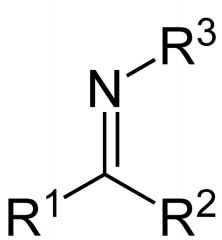
Imine. It is made by reacting a primary amine with an aldehyde or ketone.
|
|
|
What is this compound? How is it made?
|
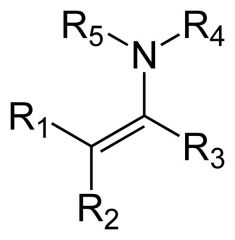
It is an enamine. "En-" for "alkene" (the nitrogen is attached to a carbon that is part of a double bond) and "amine" for the amine group. It is made by reacting a secondary amine with an aldehyde or a ketone.
|
|
|
What is this and what is it used for?
|
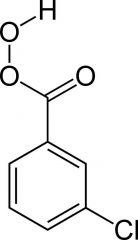
mCPBA (meta-chloroperbenzoic acid)
It is used to convert alkenes to epoxides. |

