![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
What is the functional group with a SINGLE bond connecting H and C?
|
Alkane
|
|
|
What is the functional group with a DOUBLE bond connecting C and C with H's attached to each C?
|
Alkene
|
|
|
What is the functional group with a TRIPLE bond connecting H and C?
|
Alkyne
|
|
|
What is the functional group for benzene? It's in a ring form, and had alternating double bonds, H is attached to each connection.
|
Aromatic
|
|
|
What is the functional group R-O-H?
|
Alcohol
|
|
|
What is the functional group R-O-R?
|
Ether
|
|
|
What is the functional group R-NH2?
|
Primary Amines
(ALL AMINES WRITTEN AS ONE WORD!) |
|
|
What is the functional group where two R groups are bonded to a N-H?
|
Secondary Amines
(ALL AMINES WRITTEN AS ONE WORD!) |
|
|
What is the functional group where 3 R groups are bonded to a single N?
|
Tertiary Amines
(ALL AMINES WRITTEN AS ONE WORD!) |
|
|
What is the functional group with a double bond between C and O, and also attached to the C is an R group and a H?
|
Aldehyde
|
|
|
What is the functional group with a double bond between C and O, with 2 R groups attached to the C?
|
Ketone
|
|
|
What is the functional group with a double bond between C and O, with a R group and an OH group attached to the C?
|
Acid
|
|
|
What is the functional group with a double bond between C and O, with a R group and an OR attached to the C?
|
Ester
|
|
|
What is the functional group with a double bond between C and O, with a R group and a N attached to the C...but there's also 2 H's on the N?
|
Amide
|
|
|
What's the classification of a double bond between C and O?
|
Carbonyl (carbon-eel)
|
|
|
IUPAC prefixes (1-12)
|
meth, eth, prop, but, pent, hex, hept, oct, non, dec, undec, dodec
|
|
|
n-butyl v sec butlyl v isobutyl v t-butyl
|
Straight chain, first carbon is secondary, second carbon is secondary, first carbon is tertiary
|
|
|
neopentyl
|
first carbon primary, second is tertiary
|
|
|
Vinyl v allyl
|
alkene substituents, for vinyl yo have a monosubstituted alkene (or a simple double bond as a substituent). Allyl subsituents are propylenes, dbl bond between c1 and c2, then attached at c3
|
|
|
nomenclature of haloalkanes
|
IUPAC names them "bromobutane" OR common name, butane bromide...
(ie haloalkane v alkyl halide) Flouro, bromo, chloro, iodo |
|
|
Diol- vicininal v geminial
|
vicinal are on adjacent carbons, geminal is 2 on the same one
|
|
|
Ether
|
-R-O-R
- alkoxy prefix. I.e. basically naming it as a subsitutent. the smaller chain is the prefix-oxy- then longer chain. Ie. methoxyethane. OR methy ethly ether - "ether" on its own refers to diethyl ether |
|
|
Oxirane
|
cyclic ether, also called epoxides
- oxirane on its own is a triangle, one c replaced by O |
|
|
THF
|
Tetrahydrofuran. cyclopentane with one C replaced by O`
|
|
|
Aldehyde
|
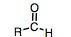
Carbonly at the end of a chain
Named with an "al" suffix |
|
|
amide
|
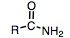
|
|
|
amine
|
-NH2
- if is the highest priority, then use "____amine" if not, "amino___" - if other groups are attached to the "N" use n-___-___ IE "n-ethly pentamine" |
|
|
formaldehyde
|
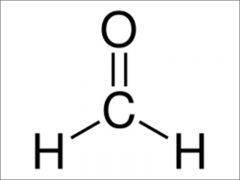
|
|
|
Acetaldehyde
|
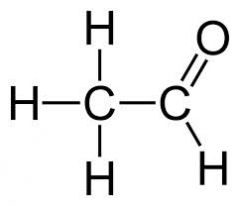
|
|
|
propionaldehyde
|
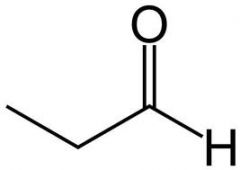
|
|
|
acetic acid
|
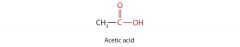
|
|
|
formic acid
|
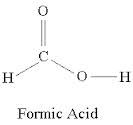
|
|
|
propionic acid
|
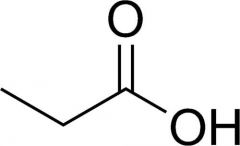
|
|
|
acetone
|
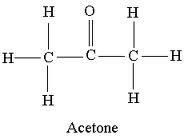
|
|
|
Ketone
|
carbonyl in the middle, "one" suffix. Ie. 2 pentanone (coarbonly at carbon 2) or list of groups followed by ketone.
if the ketone is a substituent, use "oxo" prefix |
|
|
carboxylic acid
|
COOH, "oic acid" suffic
|
|
|
ester
|
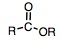
"alkoxy carbonly" or "oate"
|
|
|
Acyl halide
|
R-C=O - X Halocarbonyl or -Oyl halide
|
|
|
nitrile/cyanide
|

Cyano-, -nitrile
|
|
|
thiol
|
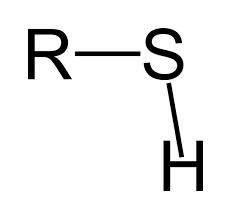
Sulfhydrl, thiol
|
|
|
imine
|
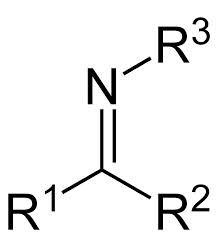
|
|
|
sulfide
|
R-S-R, alkylthio-alkane
|
|
|
nitro
|
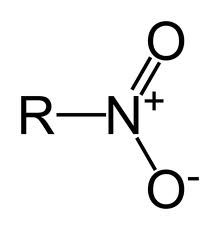
nitro
|
|
|
azide
|
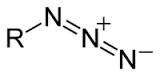
azido-
|
|
|
diazo
|
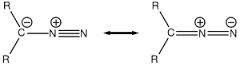
diazo
|

