![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
77 Cards in this Set
- Front
- Back
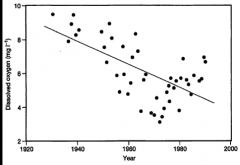
What are some possible explanations for this trend through time? |
- Increased temp could decrease the amount of oxygen that can be in the water and could change the seasonal mixing - Increased duration of stratification could cause |
|
|
What are three reasons DIC (dissolved inorganic carbon) are important in freshwater systems? |
1) Main source of C for photosynthesis |
|
|
Nutrient |
- Any element that an organism must acquire to live, grow, and reproduce. Energy for metabolism. |
|
|
What are the 6 big macronutrients? |
C, H, N, O, P, S (sounds like chinops) |
|
|
Biogeochemical cycle |
- Is a pathway by which a substance moves through biotic (biosphere) and abiotic (lithosphere, atmosphere, and hydrosphere) realms. |
|
|
Name these: CO2 H2CO3 HCO3- CO32- CaCO3 Ca(HCO3)2 |
- carbon dioxide - carbonic acid - bicarbonate - carbonate - calcium carbonate - calcium bicarbonate |
|
|
What adds up to give you the sum total of CO2 (DIC)? |
ΣCO2 (DIC) = CO2 + HCO3- + CO32- |
|
|
Alkalinity |
- Amount and types of compounds that collectively increase the pH of water or acid neutralizing capability. Expressed as the total quantity of a base. |
|
|
Buffering capacity |
- Is the ability to resist pH changes. |
|
|
Lake water pH is largely controlled by what? |
- The buffering reactions of ionic forms of DIC: H2CO3 = carbonic acid, HCO3- = bicarbonate, and CO32- = carbonate. - These compounds are largely derived from the weathering of rocks |
|
|
Endorheic regions |
- Basins that are closed hydrologic systems, they don't flow into the ocean. - Soda lakes found in these regions have high pH because they contain high [Na2CO3] due to the inability to drain to the ocean, so are controlled mainly by precipitation |
|
|
Distribution of ΣCO2 (DIC) in water is influenced by what 5 factors? |
1) Atmospheric CO2 and solubility in water 2) Equilibrium reactions 5) Respiration (source) |
|
|
How soluble is CO2 in water compared to O2? |
- CO2 is very soluble in water and lakes tend to be supersaturated - 200x as soluble as oxygen in water |
|
|
How is the solubility of CO2 controlled? |
- Inversely related to temp and salinity - Directly related to pressure - Influenced by ionic carbon complex |
|
|
How does the addition or removal of CO2 to a system change the equilibrium and pH? |
- CO2 input shifts equation to the right and decreases pH - CO2 removal by photosynthesis shifts equation to the left and increases pH |
|
|
Lake Whiting |
- A massive calcium carbonate precipitation event - The precipitation of CaCO3 (marl) occurs when high photosynthesis and temp (great losses of CO2) shifts the DIC equilibria. |
|
|
Why study [P] and cycling? |
- Key component of biomolecules (e.g. DNA, RNA, ATP, lipids) - Often first element to limit algal growth - Management implications (Detergent Wars of the 1960-70s) |
|
|
Orthophosphate |
PO4-3 - Is the most significant form of P. P is very reactive and tends to form insoluble compounds- especially under aerobic (oxidized) conditions. - Is the most important, and the form that algae use, it is difficult to measure reliably |
|
|
Liebig’s Law of Minimum |
- The element present in the lowest [conc.] relative to its demand limits growth. |
|
|
Redfield ratio |
- Rapidly growing algae uptake ratios of C, N, and P in a characteristic proportion, 106C: 16N: 1P (by atom), |
|
|
Luxury uptake |
- In order to obtain the limiting resource (P), algae can store P in vacuoles when availability is high |
|
|
Explain each part:
Total Phosphorus (TP) = DIP + DOP + PP
|
DIP – (<5%) dissolved inorganic phosphorus PO43- DOP – organic colloids, less available |
|
|
[TP] in natural waters ranges from: |
< 1 ug l-1 to > 200 ug l-1 - Variation is high and reflects regional geology. - Most NB lakes 10-50 ug l-1 |
|
|
Trophic status of an aquatic ecosystem |
- Represents its level of nutrients and algal growth. |
|
|
Explain how phosphorous would change in lakes with an orthograde O2 curve vs lakes with a clinograde O2 curve. |
- Lakes with orthograde O2 curve show little variation in P with depth. - Lakes with clinograde O2 curve show ↑ P in hypolimnion. - P exchange regulated by oxygen supply and metabolism of decomposers. - Sediment-water interface is key. |
|
|
External (allochthonous) phosphorous sources |
1) Atmospheric fallout (eg dust from erosion, fires, fossil fuels) 2) Ground water- very minor 5) Guano from animals (eg seabirds) |
|
|
Internal (autochthonous) sources of P in lakes
|
1) Food web- excretion by zooplankton and fish (taken up quickly) 2) Algae and macrophytes 3) Sediments and benthos |
|
|
Name transport mechanisms of P at the sediment-water interface. |
- Diffusion - Wind-induced turbulance - Bioturbation - Gas ebuilition - Attached algae/BG's - Rooted aquatic plants |
|
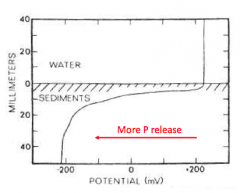
Explain |
- If you have an oxidized sediment-water interface, then there is a barrier to PO43- release from the sediments. |
|
|
What happens to P movement when sediment-water interface is anoxic? |
- If the interface goes anoxic, then the barrier erodes and you get internal loading of PO43- from the sediments. - As redox potential ↓ = release of P, Fe, etc. increase markedly. |
|
|
Iron (Fe) role interacting with P |
- Iron (Fe) plays a key role as a binding agent under oxic conditions. - Fe (III) oxide (ferric form) binds to orthophosphate preventing its release from the sediments. |
|
|
How do we manage excess nutrient loading? |
- Removal of P from wastewater in sewage treatment plants - Prevent wastewater from entering rivers and lakes - Buffer strips along nearshore areas to trap nutrients - Add chemical agents that bind P |
|
|
Why study N and its cycling? |
- Biomolecules (e.g. proteins, DNA, chlorophyll) largely composed of N - Major nutrient in all ecosystems - Complex microbial-driven cycle - Human activities adding huge amounts to biosphere - N accumulating in global ecosystems! |
|
|
N enters a drainage basin in many forms: |
1) N2 gas (strong bonds) *(only available to primary producers when converted into ammonia NH3) 2) Inorganic N (all can be found in atmosphere) NH4+ = ionized ammonia NH3 = ammonia NO2- = nitrite NO3- = nitrate 3) Organic N DON = dissolved organic N PON = particulate organic N |
|
|
Major transformations of N are: |
- N fixation (N2 > NH3, anoxic conditions) - Nitrification (NH3 > NO2- > NO3-, oxic) - Denitrification (NO3- > NO2- > NO/N2O . N2, anoxic) - Anammox ( NO2- and NH3 > N2, anoxic) - Ammonification (organic N > NH3, oxic) |
|
|
Sources of N in aquatic ecosystems |
1) Direct deposition onto surface (NOx, mainly forms) 2) Allochthonous inputs from surface and groundwater interacting with soils 3) N fixation in water and sediments |
|
|
N fixers in aquatic systems |
- Cyanobacteria (blue-green) and heterotrophic bacteria (often found in sediments and water column) - All N fixers have an enzyme complex called nitrogenase that catalyzes the reduction of N2 to NH3 (ammonia). |
|
|
Heterocyst |
- Cell in cyanobacteria where N fixation occurs. - Light-dependent process. - Protect enzyme from O2. |
|
|
Nitrification |
- Conversion of ammonia to nitrite and then to nitrate. - Occurs aerobically by microbes. - First is oxidation of ammonia to nitrite via hydroxyamine. - Next is the oxidation of nitrite (NO2-) to nitrate (NO3-) by different set of bacteria. |
|
|
Anammox bacteria |
- Oxidize ammonia in anaerobic conditions by using nitrite as the electron acceptor to produce N2. |
|
|
Denitrification |
- Conversion of nitrate to N2, thus removing bioavailable N and returning it to the atmosphere. - Necessary in sewage treatment. - It is an anaerobic process, occurring in soils, sediments, and anoxic zones of lakes. |
|
|
Losses of N in aquatic systems
|
1) Outflow from surface and groundwater
2) Denitrification by bacteria (NO3- to N2) - returns N2 back to atmosphere in gas form 3) Sedimentation of inorganic and organic N compounds *Also annamox |
|
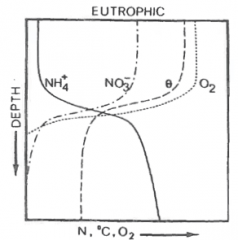
Explain |
- NH4+ quickly utilized by algae in upper layers - Increase in NH4+ at thermocline because of anoxic processes - During anoxia release of NH4+ occurs from the sediments. Nitrification ceases. |
|
|
What’s so special about shallow lakes? |
- Numerically dominant in lowland regions - Humans depend on these systems - High biodiversity where wetland and littoral habitat coincide - Stability (?) > Alternative stable states |
|
|
Shallow lake (pond) |
- Permanent standing body of water where light penetrates to the bottom sediments- often, macrophytes cover the entire basin. |
|
|
List some general characteristics of shallow lakes. |
- Extensive littoral system - Organisms can use macrophytes as cover from threats - Different predation dynamics - Important for waterfowl - More simple food webs |
|
|
Origins and distribution and shallow lakes. |
- Occur in abundance in lowland areas of gentle relief. - Basins often formed by: glacial activity, river erosion (or movement), and wind deflation. - Humans have created many for agriculture, industry, water storage, and recreation. |
|
|
Characteristics of shallow lakes
|
- Nutrient loading is high (lowlands and small volume). - Nutrient losses less and recycling occur faster- results in light availability regulating primary production. - Macrophytes and periphyton dominate → habitat heterogeneity can lead to high biodiversity. - No distinct hypolimnion and stratification is weak (polymictic). |
|
|
Relationship between macrophytes and macroinvertebrates/fish |
- Increase in macrophytes means more macroinvertebrates and more fish |
|
|
Factors that maintain macrophyte dominance
|
- Reduced wave action or currents (e.g. geomorphic conditions) - Uptake of P and N by submersed macrophytes - Large surface area for periphyton/epiphyton habitat - High metabolism of macrophytes (e.g. restrict fish populations) |
|
|
Daphnia sp. |
- Key invertebrate grazer of phytoplankton - Filter water to obtain algal food - Can reach >100 individuals/ L - Vulnerable to fish/invert predation - Adapt in many ways to predation |
|
|
Factors that maintain phytoplankton dominance
|
- Reduced grazing from zooplankton due to lack of refugia - Suspension of sediments = ↓ light and poor macrophyte substrate - Strong competition by algal & cyanobacteria - Once established, high nutrient loads and dense phytoplankton tend to persist |
|
|
How can habitat size and isolation can promote species richness in small lakes? |
1) Richness ↑ with lake size, 2)Shallow lakes tend to be in either a fish-dominated, turbid state, or in a state with high macrophyte biomass, 3)Biodiversity is higher in a fish-poor vegetated lake than in a fish-dominated unvegetated lake of the same size, and 4) The occurrence of the vegetated state decreases with lake size. |
|
|
Other control mechanisms on plant dominance in shallow lakes. |
- Fish introduction: species that disturb the sediments prevent macrophytes from rooting - Waterfowl and muskrat: voracious herbivores - Humans: herbicide application, mechanical cutting, and biomass removal |
|
|
Role of macrophytes in lakes |
- Macrophytes influence the physical, chemical, and biological composition of lakes. - When macrophyte biomass is lost there can be: • Change in habitat |
|
|
Indicators of shifting alternative states |
Far from threshold: • Low variability Approaching threshold: • High variability • Low recovery rate |
|
|
Factors that may determine if shallow lakes are clear or turbid
|
- Nutrient concentrations - mainly [P] - Zooplankton populations - Macrophyte biomass - Climatic controls of water depth |
|
|
Trophic cascades |
- Can occur when predators reduce the abundance or alter behaviour of prey, thus releasing the next lower trophic level from predation creating cascading effects down the food chain |
|
|
Sedimentation |
- Is the key process to ontogeny- controlled by within-lake and catchment loadings. |
|
|
Succession |
- Phenomenon of change through time of plant communities, usually after disturbance. |
|
|
Lake ontogeny (NOT succession) |
- The developmental history of lentic waters. |
|
|
Factors that maintain low-nutrient conditions |
1. Negligible loading 4. Oxic hypolimnion |
|
|
Trophy |
- The rate at which organic matter is supplied by or to a lake per unit time. - Dystrophy “dark-water lakes” - Oligotrophy - Eutrophy |
|
|
Dystrophic shallow pond |
- Acidic and dark water |
|
|
Characteristics of wetlands (bog, fen, marsh, swamp, peatland) |
Transition between aquatic and terrestrial ecosystems Water-saturated long enough to promote aquatic processes Water table near surface & poorly drained soils Specialized biota adapted to these conditions Very productive ecosystem |
|
|
Importance of wetlands |
“Kidneys” of the landscape Flood storage & mitigation Protect against erosion Important habitat and C sink |
|
|
Indicators of environmental change |
- Plant macrofossils (vegetation change) - Pollen (vegetation change) - Protozoa (moisture levels) - Phytoliths (disturbance) |
|
|
Transition from a shallow lake (pond) to a terrestrial system |
- Characterized by accumulation of organic matter in excess of degradation. - Does not happen overnight, can take thousands of years. - Shift in origin of nutrients from catchment to atmosphere. |
|
|
Sphagnum overstory |
- Waterlogged environment - Mosses favoured: accumulation of nutrients in non-available form, vascular plants restricted - Anaerobic conditions: reduced decomposition & nutrient availability, peat accumulation |
|
|
Rheotrophic |
- Nutrients derived from surface or groundwater |
|
|
Ombrotrophic |
- Nutrients derived from atmospheric sources |
|
|
Quaking bogs |
- Develop in deep basins of small surface area - Mat encroachment toward the centre of the basin overlies littoral peat accumulations and much of the open water |
|
|
Ontogeny results in water chemistry shifts |
Rheophilous to Ombrophilous - Large decrease in pH - Increase in H - Decrease in HCO3 - Decrease in salinity |
|
|
Charismatic megaflora of the Bacillariophyceae
|
- Didymosphenia geminata (aka rock snot, Didymo) - Benthic, colonial diatom native to Northern Hemisphere - Clear, low-nutrient rivers |
|
|
Didymo stalk production results from what? |
- Low inorganic [P] |
|
|
Didymo impacts to habitat and higher trophic levels |
Alter foodwebs Change river structure/function - substrate - aesthetics |
|
|
Conditions favourable to Didymo that may promote blooms
|
1) Early ice-out extends growing season - warmer/milder winter - potential to change way ice leaves, often scrapes bottom and disturbs bottom 3) Base-flow period extended 5) Also hypothesized: N deposition, improved land-use, and fewer marine-derived nutrients - N increases plant growth, so more plants would pull more P out of the soils, leaving |

