![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
- 3rd side (hint)

Boyle's Law |
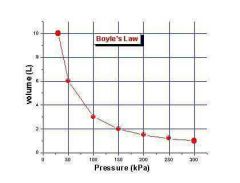
The volume of a given amount of gas is inversely proportional to the pressure at a constant temperature and mass. |

What happens to fishes when they are caught from the ocean? |
|

Charles' Law |
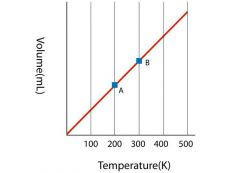
The volume of a given amount of gas is directly proportional to the Kelvin temperature at a constant pressure. |

What may happen to an inflated balloon as it is brought outside? |
|

Gay-Lussac's Law |
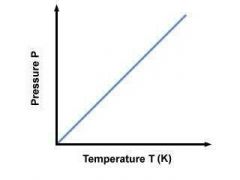
The pressure of a gas of fixed mass and fixed volume is directly proportional to the gas' temperature in Kelvin. |

What might happen if you throw a can of aerosol into a fire? |
|
|
Isothermal |
A change of system in which the temperature remains constant. |
Thermal |
|
|
Isobaric |
A change of system in which the pressure remains constant. |
Baric |
|

Avogadro's Law |
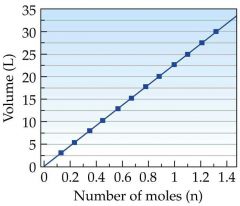
The volume of a given amount of gas varies directly with the number of moles at constant temperature and pressure. |
Lungs expand as they fill with air. |
|

What is the equation shown in the Combined Gas Law? |
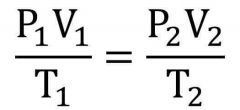
|
|
|
Ideal Gas Law |
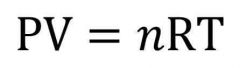
A law describing the relationship of the measurable properties of an ideal gas. |
|

