![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
103 Cards in this Set
- Front
- Back
|
Draw the chemical structure of a triglyceride. |
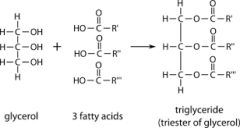
|
|
|
Provide the short name forlinolenic acid (N:M format) and then draw this fatty acid structure. |
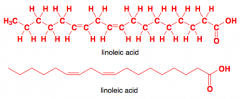
18:3n3 |
|
|
In plant oils, in whatlocation on the triglyceride is this fatty acid (linolenic acid) most often found? |
SN2 |
|
|
Describe two structural or functional differencesbetween saturated and unsaturated fatty acids. |
-Saturated fatty acids have no double bonds between molecules while unsaturated do. -Saturated fatty acids acids have all of their available electron donor and acceptor spots filled with hydrogens, only some spots of unsaturated are filled. - Saturated fats are solid at room temp, unsaturated are liquid |
|
|
What does the terminology omega-3 fatty acidmean? |
Omega-3 fatty acids: A class of essential fatty acids found in fish oils, especially from salmon and other cold-water fish, that acts to lower the levels of cholesterol and LDL (low-density lipoproteins) in the blood. (LDL cholesterol is the "bad" cholesterol.)EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are the two principal omega-3 fatty acids. XXX |
|
|
Describe two common structural changes thatoccur in lipids during hydrogenation and how these influence the Tm of thelipid. |
– Saturated bonds and Cis to trans conversion - This leads to higher melting tempeatratures |
|
|
When comparing butyric, stearic, oleic, andlinoleic fatty acids, identify which has the highest Tm and describe why thisis so. |
Stearic has the highest melting point because it is saturated and it has the largest chain. This is so because it it saturated and has more carbons than the others. |
|
|
Identify and describe the 3 main types of lipidcrystal structures. Which is most stable? Which has the highest melting temperature? |
Simple(Nonpolar esters of fatty acids with alcohols), compound (Lipids that contain fatty acids and anothercompound) and derived(Mixed group of lipids). Simple is the most stable. Simple has highest melting temp |
|
|
What characteristics of phospholipids andmonoglycerides contribute to their function as emulsifiers? |
Phospholipids act as emulsifiers because they can surround droplets of oil, allowing them to remain suspended in a watery environment. |
|
|
Interesterification |
Also called randomization• Def. Rearrange fatty acids so they becomeevenly distributed randomly amongtriacylglycerol molecules of the fat -To improve consistency and usefulness |
|
|
Explain thedifferences in melting points between Caproic and stearic acids |
Stearic is about 70 C, Caproic is much loweer |
|
|
What form(liquid or solid) would you expect the following food lipids to demonstrate at4ºC, 21ºC, and 37ºC? Why? Coconut lipid Salmon lipid Milk lipid Chicken lipid Olive lipid |
Coconut - solid, solid, liquid Salmon - liquid, liquid, liquid Milk- solid, solid, liquid Chicken - solid, solid, solid Olive- liquid, liquid, liquid |
|
|
Describe the influences ofchain length, crystal structure, and degree of unsaturation on the meltingpoint of lipids |
Longer and straighter the chains:the higher the MP As degree of unsaturation increases:MP decreases- Oils: high content of unsaturated FA Trans FA have:higher MP than cis (pack more tightly) |
|
|
Describe thecharacteristics of alpha, beta, and beta prime lipid crystals |
Beta crystals higher MP than beta prime, alpha crystals lowest MP (dueto less crystalline and more disordered /randomstructure) |
|
|
How do emulsifiers decrease interfacial tension? |
With the help of surfactants. It mediates interactions between hydrophobic and hydrophilic interactions |
|
|
Hydrogenation is one of themost prevalently used chemical reactions in the fat processing industry. What factors influence this reaction? |
• Increases theresistance tooxidation and flavordeterioration • Increases the meltingpoint of the oil/fat |
|
|
Explain the differences in melting points between palmitic and palmitoleic acids |
palmitic is about 64 degrees C, palmitoleic acid is 1 degree C |
|
|
Explain the differences in melting points between n-octadecanoic, cis-9-octadecenoic and trans-9-octadecenoic acids. |
n-octadecanoic is Stearic acid and 70 degrees c, cis-9-octadecenoic is 6 degrees C, trans-9-octadecenoic is 37 degrees C |
|
|
Polymorphism |
Polymorphs are different crystal forms of the same chemical substance. Transformation of one polymorph into another can occur with orwithout melting the solid. |
|
|
Primary attractive force between nonpolar lipids? |
Van der Waals |
|
|
Whatare the four main bonding forces present within and between proteins? For each type of bond, give an example of oneamino acid with a side chain that can participate in that type of bond. |
Peptide bond, hydrogen bond, covalent bond, ionic bond. XXX |
|
|
Describethe pKa of an ionizable amino acid and why it is important forprotein structure and function in a food |
Proteins are more stable against denaturation at theirisoelectric point than at other pHs XXX |
|
|
Whatis the common covalent crosslink found between proteins? Explain how and why this crosslink is oftenbroken and reformed during processing. |
- Important in stabilizing structure; intra- andintermolecular bonding – Sulfhydryl-disulfide interchange reactions important inbreadmaking and other food applications. XXX?Generally, when a protein solution is heated to a progel stateand then cooled, interactions take place |
|
|
Defineand describe protein denaturation. Someproteins are heat-stable and resistant to being denatured. In terms of amino acid composition orstructure, propose one explanation for heat-stability. |
- Any major modification in theconformation (2, 3, or 4o) not accompanied by disruptionof the primary structure (peptide bonds); - Ultimatelyresulting in total unfolding of the protein Heat – causes sharp transition from native to denaturedstate (can measure as a thermal transition). Hightemperatures favor hydrophobic interactions.Susceptibility of proteins depends on many factors -nature of the protein, protein concentration, water activity,pH, ionic strength, kind of ions present |
|
|
Ifyou were designing a protein to have good solubility properties, what are 2aspects (amino acid types, or 2o or 3o structures) thatyou would incorporate into the protein structure? |
Casein is an example of a protein that exists unfolded and isstable to most denaturants, including heat XXX |
|
|
Whydo most proteins lose some of their solubility characteristics at theirisoelectric point (pI)? Why doesaddition of heat usually enhance precipitation at a proteins pI? |
No electrostatic repulsion Proteins may aggregate and precipitate XXX |
|
|
What makes aprotein a good emulsifier? |
|
|
|
Why woulddeamidation of glutamine and asparagines increase water solubility? |
Glutamine residues may be acted on by an enzyme calledtransglutaminase, which catalyzes the cross-linkingbetween glutamine and a primary amino group.? |
|
|
What islysinoalanine and briefly explain how it forms? |
• lysinoalanine formed from lysine anddehydroalanine – lose lysine availability andis toxic |
|
|
You have a soyprotein isolate (~90% protein content). How would you go about making texturized soy protein? |
Thermal coagulation and film formation – e.g.,concentrated soy protein solutions, hydrophobiccereal prolamin proteins |
|
|
From starchstarting material, name 2 enzymes that are used to make high-fructose cornsyrup |
|
|
|
What is theaction of lipase, and for what is it used in the industry? |
Catalyst = enzyme lipase |
|
|
What 2 reactantsare required for the Maillard browning reaction? |
Amino containing compound and reducing sugar |
|
|
What enzyme catalyzeshydrolysis of fatty acids from the glycerol molecule? |
enzyme lipase |
|
|
What does the food industrydo to prevent hydrolytic rancidity from occurring in processed foods? |
• Lower temperature• Inactivate enzymes• Choose fats for formulations carefully– Short chain fatty acids (<12C) arehydrolyzed faster than longer chain |
|
|
How do emulsifiers decreaseinterfacial tension? |
The system is stabilized by an emulsifier thatslows the separation rate of the dispersed phase |
|
|
Why are polyunsaturated fatty acids more susceptible to autoxidation than saturated fatty acids? |
Double bonds? |
|
|
What four physicalproperties of lipids affect the crystallization of a food fat? Explain these relationships. |
Mechanical properties, mouthfeel, physical stability, visual apperance |
|
|
Hydrogenation is one of themost prevalently used chemical reactions in the fat processing industry. What factors influence this reaction? |
Def: a reaction betweenhydrogen gas and unsaturatedfatty acids in the presence ofcatalysts (nickel, platinum,palladium) that results in amore saturated fatty acid |
|
|
Define and givetwo examples of each of the traditional classifications of proteins. |
CompositionHomo/hetero(conjugated)-protein ConfigurationFibrous/globular SolubilityAlbumin/globulin/glutelin/prolamine/etc Sourcemeat/milk/egg/plant |
|
|
List the amino acids thathave charged and polar side chains. Draw their structures. Do the same for those that have uncharged andpolar side chains and those that have nonpolar side chains. |
Charged and Polar AA• Aspartic acid, glutamic acid, • lysine, arginine, histidine Uncharged and Polar AA• Asparagine, glutamine, cysteine, glycine, serine,threonine, tyrosine SOLUBLE IN WATER |
|
|
What is the primarystructure of a protein? What bonding force(s) maintain(s) this structure? Whatdetermines this structure? |
Linear sequence of amino acids joined bypeptide bonds Partial double-bond of peptide bond hasstructural implications Resonance structure precludes protonation ofthe peptide N-H group |
|
|
What is the secondarystructure of a protein? What bondingforce(s) maintain(s) this structure? |
Spatial structure the polypeptide chainassumes along its axis (due to sequence ofAA and side chain structures) α-helix, β-sheet, β-turn |
|
|
What is the tertiarystructure of a protein? What bonding force(s) maintain(s) this structure? Whatdetermines this structure? |
3-dimensional organization of thepolypeptide chain Covalent bonds stabilize tertiarystructure Most importantly is the relocation of the hydrophilic residues,especially the charged residues, at the protein-water interface |
|
|
Whatis the quaternary structure of a protein? What bonding force(s) maintain(s)this structure? |
Association of more than onepolypeptide chain, known assubunits, to form dimers,trimers, tetramers… structure is determined by – electrostatic interactions – hydrogen bonding – hydrophobic interactions – disulfide bonds – between the R groups of aminoacids that are in contact on thesurfaces of the associatingprotein chains |
|
|
Define denaturation of aprotein. Why is it important to the foodscientist? |
Any major modification in theconformation (2, 3, or 4o) not accompanied by disruptionof the primary structure (peptide bonds); ultimatelyresulting in total unfolding of the protein Effects include: – Decreased solubility resulting from unmasking ofhydrophobic groups – Altered water-binding capacity – Loss of biological activity – Increased susceptibility to attack by proteases– Increased intrinsic viscosity – Inability to crystallize. |
|
|
What are some of the functional propertiesresulting from protein manipulation and processing? |
1. Hydration (or hydrodynamic) properties(dependent on protein-water interactions)– Related to water absorption and retention• wettability• swelling• adhesion• dispersibility• solubility• viscosity (thickening) 2. Related to protein-protein interactions– precipitation– gelation– formation of various other structures (doughs, etc.) 3. Surface properties– surface tension– emulsification– foaming |
|
|
What determines the tertiary structure of aprotein? What characteristics aredetermined by the tertiary and quaternary structure of a protein? |
the relocation of the hydrophilic residues,especially the charged residues, at the protein-water interface free energyof the molecule is reduced to the minimum value possible |
|
|
What determines the solubility of aprotein? Specifically, how is solubilityimpacted by pH, salt concentration and protein denaturation? |
Solubility may be increased in a protein by: – Changing pH – Low level of salt – Low level of heat (to 40oC) – Partial hydrolysis – |
|
|
Define the term gel. What types of gels are usually found in foodsystems? How can we differentiatebetween these two gel systems? |
an intermediate phase between a solid and liquid– a “substantially diluted system which exhibits nosteady state flow” Thermally reversible gels are sustained primarily bynoncovalent interactions (gelatin) Because hydrophobic bonds are strong at hightemperatures, these gels are generally thermallyirreversible (egg-white gels) |
|
|
Listand discuss the factors that influence water binding capacity of proteinsolution. |
At low concentrations (<0.2 M), salts increasethe water binding capacity of proteins Partial hydrolysis leads to Increased water binding andemulsification properties Protein denautration alters water-binding capabilities |
|
|
What is an emulsion? How are proteins involved in forming a stableemulsion? What makes a protein a goodemulsifier? |
• Emulsions are the macroscopic dispersions of twoimmiscible liquids ROLE OF PROTEINS ON FOAMING& EMULSIFICATION• Lowers surface & interfacial tensions dueto adsorption at the interface- ease of formation of foams & emulsions• Stabilizes foams & emulsions |
|
|
What properties of aprotein cause it to have good foaming properties? |
Lowers surface & interfacial tensions dueto adsorption at the interface Foamability of different proteins depend ontheir rates of adsorption and their ability tounfold at the air-water interface Solubility of protein– More soluble form better foams • Ability of protein to:– Rapidly diffuse and adsorb at the interface– Rapidly unfold (denature) at the interface– Stabilize (via intermolecular interactions) in a cohesive film at theinterface • Surface hydrophobicity of protein– Hydrophobic regions of protein orient towards nonpolar air phase • Partial denaturation– More easily unfolds at air interface • Presence of lipids– May interfere with foam formation (0.03% egg yolk in egg white) • Processing parameters (energy input, shear, time) |
|
|
Define emulsion. Describethe characteristics of a good emulsifier. |
• Emulsions are the macroscopic dispersions of twoimmiscible liquids Behavior of protein at interfaces (O/W & A/W)• Interfacial behavior depends on physicalinteractions- primary structure as well as conformation- denaturation Factors Affecting Protein-BasedEmulsions• Temperature• Salts• Processing parameters– Energy input (shear), droplet size, lipid type,temperature• Protein– Solubility, surface hydrophobicity and charge,flexibility, elasticity, ease of unfolding• External phase– viscosity |
|
|
Define foam. Describe thecharacteristics of a good film forming protein. |
a mass of small bubbles formed on or in liquid, typically by agitation or fermentation. Factors Affecting Foaming Solubility of protein– More soluble form better foams• Ability of protein to:– Rapidly diffuse and adsorb at the interface– Rapidly unfold (denature) at the interface– Stabilize (via intermolecular interactions) in a cohesive film at theinterface• Surface hydrophobicity of protein– Hydrophobic regions of protein orient towards nonpolar air phase• Partial denaturation– More easily unfolds at air interface• Presence of lipids– May interfere with foam formation (0.03% egg yolk in egg white)• Processing parameters (energy input, shear, time) |
|
|
Define gel. Describe thecharacteristics of a good gel forming protein. |
A gel is an intermediate phase between a solid and liquid– a “substantially diluted system which exhibits nosteady state flow” Will gel in hot and cold |
|
|
Write the equation forStoke’s Law. Define each term. |
V = (2gr^2(d1-d2))/(9pi^2) |
|
|
Define each of thefollowing heat-induced changes in proteins. Identify which are common and whichare uncommon in food products.1. denaturation2. aggregation3. gelation4. precipitation5. thermal degradation6. hydrolysis7. oxidation10. maillardbrowning |
1. Denaturation - Losing 2,3,4 structure and unfolding to end up at highest energy level state. |
|
|
Describe the heat induced changes in the following proteinfunctional properties:1. hydrophobicity2. solubility3. emulsifying capacity4. gelation capacity5. foaming properties6. enzyme activity |
Emulsifying Capacity:– Measured as the volume of oil that can be emulsifiedper gram of protein in an oil-in-water (O/W) system Hydrophobicity– Surface hydrophobicity of proteins mayincrease or decrease after heating Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent Gelation Capacity:– The amount of water that can be bound ortrapped per gram of protein Enzyme Activity:– Temperature controls the rate of enzymecatalyzedreactions and influences enzymestability |
|
|
You are working in R&Dfor a major food company and have been assigned the task of improving thequality of your protein-based foam/emulsion/gel product because the product isphysically destabilizing before end of shelf-life What ingredients are used to stabilize thefoam/emulsion/gel? How do they work? |
Gel: Some gels are also stabilized by disulfide crosslinks Emulsion : Two phases will rapidly separate unless there is anadsorbed surfactant present at the interface to stabilize itby protecting against close contact and association ofindividual globules Foam: Soluble cereal proteins stabilize bubbles (gas cells)before starch gelation stabilizes the crumb structure |
|
|
You are working in R&Dfor a major food company and have been assigned the task of improving thequality of your protein-based foam/emulsion/gel product because the product isphysically destabilizing before end of shelf-life What might be causing thedestabilization of your foam/emulsion/gel? |
Heat |
|
|
Lipids in foods are most often structured as free fatty acids. True or False? |
False. Triglycerides
|
|
|
Saturated fatty acids contain double bonds connecting carbons. True or false? |
False. Only unsaturated have double bonds
|
|
|
Trans fatty acids are always unsaturated. True or false? |
True
|
|
|
Type of chemical bond that links fatty acids to glyceride to form lipids? |
Ester bonds
|
|
|
Compared to starch, lipids are... |
Smaller, hydrophobic |
|
|
In fatty acid chains, define N:M
|
N = Carbon M = Double Bonds |
|
|
Omega naming system for fatty acid # carbons from double bonds, label from carboxy end. True or False? |
False, From Methyl end |
|
|
Most common fatty acid in foods |
Oleic (18:1)
|
|
|
Trans fatty acids are more linear than cis fatty acid counter parts True or false? |
True
|
|
|
Milk fats are unique because... |
Contains shorter chain fatty acids than other animal fats
|
|
|
Lipids from plant sources generally have unsaturated fatty acids in SN2 position
True or false? |
True |
|
|
Fully hydrogenated fats contain trans fatty acids True or False |
False
|
|
|
In an o/w (Oil in water) emulsion, continuous phase is.... |
Water, oil is the disperesed |
|
|
Butter is what kind of emulsion |
Water in oil
|
|
|
Stokes Law describes |
Emulsion stability, Creaming rate velocity
|
|
|
Increasing viscosity of continuous phase in an emulsion slows rate of separation
True or False |
True
|
|
|
Increasing attractive van der waals forces between fatty acids increase melting temp True or false? |
True |
|
|
Why does steric acid have the highest melting temp?
|
Longest, no kinks, most van der waals attraction
|
|
|
Solid-State transformation of one lipid crystal type to another is called... |
Polymorphism |
|
|
Polymorphism can occur even if lipids don't melt True or false? |
True
|
|
|
Hydrolytic rancidity is caused by... |
Lipase
|
|
|
Identify the statement related to hydrolytic rancidity |
Fatty Acids are cleaved off of glycerol molecule
|
|
|
Identify Rancidity traits |
Presence of lipase speeds up, reaction end products are hydroperoxides |
|
|
Hydrolytic & oxidative rancidity results in same changes to lipid structure True or false? |
False
|
|
|
Presence of oxygen can contribute to... |
Lipid oxidation |
|
|
Compare monounsaturated fatty acids to poly unsaturated acid |
Have lower Tm, oxidate more quickly |
|
|
Initiation of lipid oxidation occurs more quickly than propagation True or False |
False |
|
|
What initiates lipid oxidation? |
Singlet oxygen, chlorophyll, lipoxygenase |
|
|
Many antioxidants are molecules that are oxidized preferentially to lipids |
True |
|
|
Peptide bonds are covalent bonds that are stronger than disulfide bonds, that are also covalent. True or false? |
True |
|
|
Charged/Polar mean it is hydrophillic True or False |
True |
|
|
Denaturation is defined as loss of primary and secondary structure True or False |
False Primary structure is not typically denatured, would be extremely difficult to do |
|
|
Alpha helicals are more stable than beta sheets,
True or false |
False |
|
|
Proteins can be denatured by |
Heat, pH, mechanical sheer |
|
|
Compared to native globular protenins, denatured proteins are |
Characterized by increased viscosity and is more digestible |
|
|
Globular proteins are more sensitive to environment than fiberous proteins True or False |
True |
|
|
Proteins are least soluble at... |
ph = pI |
|
|
pH lower than pI leads to what charge? |
Positive |
|
|
pH higher than pI leads to what charge? |
Negative |
|
|
Proteins from different sources have different structures and properties True or False |
True |
|
|
Protein bond strength |
Highest to lowest - Amine - Disulfide - Hydrogen - Ionic - Hydrophobic - Van der Waals |
|
|
Identify one mech by which an emulsion can be stabilized |
By increasing viscosity of continuous phase |

