![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
118 Cards in this Set
- Front
- Back
|
Normal body fluid compartments |
- men = 60% of body weight, women = 50% of body weight
- percentage of TBW decreases with age and increasing obesity (TBW decreases because fat contains very little water) |
|
|
Distribution of Total Body Water
|
ICF- 2/3 of TWB- 40% of body weight- the largest proportion is in skeletal muscle mass
ECF- 1/3 of TBW- 30% of body weight (plasma is 1/3 of this and interstitial fluid is 2/3 of it)q |
|
|
Normal water intake
|
1500 mL in fluids PO per day, 500mL in solids or product of oxidation
|
|
|
Normal water/fluid output in one day
|
- From 800-1500 mL in urine/day is the normal range. Minimum urine output to excrete products of catabolism is 500-600 mL/day, assuming normal renal concentrating ability
- Output of 250mL/day occurs in the stool - From 600-900mL/day in insensible losses-- this is highly variable and increases with fever, sweating, hyperventilation, tracheostomies (unhumidified air) |
|
|
What 2 things does fluid shift depend on?
|
hydrostatic and oncotic pressures-- remember Frank-Starling
|
|
|
Assessing volume status
|
** This is not simple-- a patient with lower extremity edema may be euvolemic or toal-body overloaded but intravascularly depleted. Skin turgor and mucous membranes are difficult to assess and not always reliable indicators of fluid status
- Tracking I and Os is not exact because of insensible losses. Monitoring urine output is very important. - Normal urine output in infants is more than 1.0 mL/kg/hr, while normal urine output in an adult is generally 0.5 - 1.0 mL/kg/hr - daily weights give an accurate assessment of trends |
|
|
What are three reasons for oliguria
|
1. Low blood flow to the kidney (assess heart)
2. Kidney problem 3. post-renal obstruction (place a foley catheter) (dehydration) |
|
|
Quick body fluid compartment rule
|
60-40-20
- TBW is 60% of body weight (50% in women) - ICF is 40% of body weight - ECF is 20% of body weight - interstitial fluid 15% and plasma 5% |
|
|
In what patients would you expect higher insensible fluid losses?
|
- fever
- burns - open wounds - sepsis * great insensible losses and greater metabolic demands - for each degree of atmospheric temperature over 37 deg C the body's water loss increases by 100 mL/day - patients with liver failure, nephrotic syndrome, or any condition causing hypoalbuminemia then to third-space fluid out of the vasculature and may have total-body hypervolemia but intravascular depletion - Patients with CHF may have either pulmonary edema or anasarca, depending on which ventricle is involves - patients with ESRD are very prone to hypervolemia |
|
|
Fluid replacement therapy- when is normal saline used?
|
- foten used to increase intravascular volume if the patient is dehydrated or has lost blood. Usually this is NOT the best option in patients with CHF unless the patient needs urgent resuscitation
|
|
|
Fluid Replacement Therapy - D5W and 1/2 NS
|
- often the standard maintenance fluid (often given with 20 mEq of KCl/L of fluid
- has some glucose, which can spare muscle breakdown, and has water to replace insensible losses |
|
|
Fluid Replacement Therapy - D5W
|
- used to dilute powdered medicines
- may sometimes be indicated in correcting hypernatremia - only 1/12 remains intravascular because it diffuses into the TBW compartment-- NOT effective at maintaining intravascular volume |
|
|
Fluid Replacement Therapy - Lactated Ringer's Solution
|
- This is excellent for replacement of intravascular volume; it is not a maintenance fluid.
- This is the most common trauma resuscitation fluid. - Do NOT use if hyperkalemia is a concern (contains potassium) |
|
|
Causes of hypovolemia
|
1. GI losses due to vomiting, nasogastric suction, diarrhea, fistula drainage, etc
2. Third-spacing due to ascites, effusions, bowel obstruction, crush injuries, burns 3. Inadequate intake 4. Polyuria- for ex DKA 5. Sepsis, intra-abdominal and retroperitoneal inflammatory processes 6. Trauma, open wounds, sequestration of fluid into soft tissue injuries 7. Insensible losses- evaporatory losses through the skin (75%) and respiratory tract (25%) |
|
|
what happens to fluid when you give hypotonic solutions like 1/2 NS or 1/4 NS
|
It initially is transferred from the ECF to the intercellular space to equilibrate oncontic pressure
|
|
|
Clinical findings in hypovolemia
1. CNS findings 2. CV findings 3. Skin findings 4-6- other findings |
1. CNS findings- mental status changes, sleepiness, apathy, coma
2. CV findings- due to decrease in plasma volume- orthostatic hypotension, tachycardia, decreased pulse pressure, decreased CVP, and PCWP 3. Skin- poor turgor, hypothermia, pale extremities, dry tongue 4. Oliguria 5. Ileus, weakness 6. Acute renal failure due to prerenal azotemia- FeNa < 1 % and/or BUN/Cr >20 |
|
|
Diagnosis of hypovolemia
|
1. Monitor UOP and daily weights. If the patient is critically ill and has cardiac or renal dysfunction, consider placing a Swan-Ganz catheter to measure CVP and PCWP
2. Elevated serum sodium, low urine sodium, and BUN/Cr > 20:1 suggest hypoperfusion to the kidneys, which is usually due to hypovolemia, although NOT always 3. Increased hematocrit - 3% increase for each liter of fluid deficit 4. The concentration formed elements in the blood (RBCs, WBCs, platelets, plasma proteins) increases with an ECF deficit and decreases with ECF excess |
|
|
Treatment of hypovolemia
|
1. Correct volume deficit
a. use bolus to achieve euvolemia. Begin with isotonic solution (LR or NS). Do NOT combine bolus fluids with dextrose or potassium as hyperglycemia and hyperkalemia may result b. Frequent monitoring of HR, BP, UOP, and weight is essential c.Maintain UOP at 0.5 to 1.0 mL/kg/hour d. Blood loss- replace blood loss with crystalloid at a 3:1 ratio 2. Maintenance fluid a. d5 1/2 NS with 20 mEq of KCL/L is most common - dextrose inhibits muscle breakdown b. calculate maintenance fluid needs from 1 of 2 formulas (see next card) |
|
|
2 methods for calculating maintenance fluids
|
1. 100/50/20 rule to calculate total daily need
- 100 mL/kg for the first 10 kg, 50 mL/kg for the next 10 kg, and 20 mL/kg for every 1 kg over over 20 - divide total by 24 to determine an hourly rate - ex: 70 kg man - 100 x 10kg, 50 X 10kg, 20 x 50kg= 2,500 mL / 24 hours = 104 mL/hr 2. 4/2/1 rule to calculate hourly rate - 4 mL/kg for the first 10kg, 2mL/kg for the next 10 kg, 1mL/kg for every kg over 20 kg - ex: 70 kg man- (4 x 10) + (2 x 10) + (1 x 50) = 110 mL/hr |
|
|
What is the most common cause of edema development?
|
- renal sodium retention for a variety of reasons
|
|
|
Signs of volume overload
|
1. Elevated JVP
2. Pulmonary rales- due to pulmonary edema 3. peripheral edema |
|
|
Causes of hypervolemia
|
1. Iatrogenic- parenteral overhydration
2. Fluid retaining states : CHF, nephrotic syndrome, cirrhosis, ESRD |
|
|
Clinical features of hypervolemia
|
1. weight gain
2. peripheral edema (pedal or sacral), ascites, or pulmonary edema 3. JVD 4. elevated CVP or PCWP 5. pulmonary rales 6. low hematocrit and albumin concentration |
|
|
Treatment of hypervolemia
|
1. Fluid restriction
2. judicious use of diuretics 3. Monitor UOP and daily weights. Consider Swan-Ganz catheter placement depending on the patient's condition |
|
|
Salt and Water Regulation
|
1. Na+ regulation is intimately associated with water homeostasis, yet it is regulated by independent mechanisms
2. changes in Na+ concentration are a reflection of water homeostasis, whereas changes in Na+ content are a reflection of Na+ content are a reflection of Na+ homeostasis 3. Disturbance of Na+ balance may lead to hypovolemia or hypervolemia and disturbance of water balance may lead to hypo or hypernatremia |
|
|
Sodium homeostasis
|
1. Sodium is actively pumped out of cells and is therefore restricted to the extracellular space. It is the main osmotically active cation of the ECF
2. An increase in sodium intake results in an increase in GFR and sodium excretion. A decline in the extracellular circulating volume results in a decreased GFR and reduction in sodium excretion. 3. Diuretics inhibit Na+ reabsorption through various mechanism in the renal tubular system. Furosemide and other loop diuretics inhibit the Na+/K+/Cl- transporter in the thick ascending loop of Henle, whereas thiazide diuretics inhibit the Na+/Cl- co-transporter in the early distal tubule. However, most NA+ reabsorption occurs in the proximal tubule. 4. A decrease in renal perfusion pressure results in activation of the RAAS system. Aldosterone increases sodium reabsorption and potassium excretion in the late distal tubules |
|
|
Water Homeostasis
|
1. Osmoreceptors in the hypothalamus are stimulated by plasma hypertonicity (usually >295mOsm/kg). Activation of these stimulators produces thirst
2. Hypertonic plasma also stimulates the secretion of ADH from the posterior pituitary. ADH binds V2 receptors in the collecting ducts, causing the synthesis of water channels and subsequent reabsorption of more water through these aquaporins 3. ADH is suppresses as plasma tonicity decreases 4. Ultimately, the amount of water intake and output (including renal, GI, and insensible losses from the skin and respiratory tract) must be equivalent over time to preserve a steady state 5. When a steady state is not achieved, hyponatremia and hypernatremia usually occurs |
|
|
Hyponatremia
1-2. definition 3. when do symptoms develop normally? what about in head injuries? |
1. This refers to too much water in relation to sodium in the serum
2. It is typically defined as plasma Na+ concentration < 135 mmol/L 3. Symptoms usually begin when the Na+ levels falls to <120 mEq/L. An important exception is inc. ICP (e.g. after head injury). As ECF osmolality decreases, water shifts into the brain cells, further increasing ICP-- therefore it is CRITICAL to keep serum sodium normal or slightly high in such patients |
|
|
1. What is the cause of hypernatremia and hyponatremia?
2. What is the cause of hypovolemia and hypervolemia? |
1. Too much or too little water
2. Too little or too much sodium |
|
|
Hypovolemic Hypotonic hyponatremia
|
- "true hyponatremia" - serum osmolality < 280 mOsm/kg
a. Hypovolemic- low urine sodium (< 10 mEq/L) implies that increased sodium retention by the kidneys is trying to compensate for extrarenal losses (e.g. diarrhea, vomiting, NG suction, diaphoresis, third-spacing, burns, pancreatitis)- of sodium containing fluids - high urine sodium (> 20 mEq/L) - renal salt loss is likely - for example, diuretic excess, decreased aldosterone (ACE inhibitors--> dec angiotensin II --> dec aldo), ATN |
|
|
Euvolemic hypotonic hyponatremia
|
- no evidence of ECF expansion or contraction on clinical grounds
- causes: SIADH, psychogenic polydipsia, postoperative hyponatremia, hypothyroidism, oxytocin use, Admin/intake of a relative excess of free water- if the patient is given D5W (or other hypotonic solution) to replace fluids, or if water alone is consumes after intensive exertion (with profuse sweating), drugs like haloperidol (haldol), cyclophosphamide, certian antioneoplastics |
|
|
Hypervolemic hypotonic hyponatremia
|
- low urine sodium- this is due to water-retaining states. The relative excess of water in relation to sodium results in hyponatremia
- CHF, nephrotic syndrome (renal failure), liver disease |
|
|
Isotonic hyponatremia (pseudohyponatremia)
|
1. an increase in plasma solids lowers the plasma sodium concentration. But the amount of sodium in the plasma is normal -- hence, pseudohyponatremia
2. This can be caused by any condition that leads to elevated protein or lipid levels |
|
|
Hypertonic hyponatremia
|
- Caused by the presence of osmotic substances that cause an osmotic shift of water out of cells. these substances cannot cross the cell membrane ans therefore create osmotic gradients
- These substances include: a. glucose - hyperglycemia increases osmotic pressure, and water shifts from cells into ECF leading to a dilutional hyponatremia. For every 100 mg/dL increase in blood glucose above normal, the serum sodium level decreases about 3mEq/L. Note that the actual sodium content in the ECF is unchanged - mannitol, sorbitol, glycerol, maltose and radiocontrast agents all do this |
|
|
How do serum sodium levels relate to glucose levels in hyperglycemia?
|
For every 100 mg/dL over normal glucose level, the sodium level drops by 3 mEq/L, however this is because of a dilutional effect-- water coming out of cells. The true serum sodium content is normal/the same
|
|
|
Clinical features of hyponatremia
|
1. neurologic symptoms predominate- caused by "water intoxication"- osmotic water shifts, which leads to increased ICF volume, specifically brain swelling or cerebral edema
- HA, delirium, irritability - muscle twitching, weakness - hyperactive deep tendon reflexes 2. increased ICP, seizures, coma 3. GI- n/v, ileus, watery diarrhea 4. CV- hypertension due to increased ICP 5. increased salivation and lacrimation 6. Oliguria progressing to anuria- may not be reversible is therapy is delayed * As compared to acute water intoxication, when hyponatremia develops gradually (over a few days), the clinical features do NOT appear until a relatively lower sodium level is reached ( < 120 mEq/L) |
|
|
Diagnosis of hyponatremia
|
1. Plasma osmolality- low in the patient with true hyponatremia
2. Urine osmolality a. low if the kidneys are responding appropriately by diluting the urine- for example primary polydispia b. elevated if there are increased levels of ADH - for example SIADH, CHF, and hypothyroidism 3. urine sodium concentration - urine Na+ should be low in the setting of hyponatremia, urine sodium concentration > 20 mmol/L is consistent with salt-wasting nephropathy or hyperaldosteronism. Diuretics may produce this as well. c. Urine sodium concentration > 40 mmol/L is consistent with but does not define SIADH |
|
|
Treatment of hyponatremia
|
1. Isotonic or hypertonic hyponatremia- diagnose and treat the underlying disorder
2. hypotonic hyponatremia a. mild (Na+ 120-130 mmol/L) - withhold free water, and allow the patient to re-equilibrate spontaneously b. moderate (Na+ 110-120 mmol/L) - loop diuretics (given with saline to prevent renal concentration of urine due to high ADH) c. Severe (Na+ < 110 mmol/L or if symptomatic) -- give hypertonic saline to increase the serum sodium by 1 to 2 mEq/L until symptoms improve. Hypertonic saline rapidly increases the tonicity of the ECF. Do NOT increase sodium concentration by more than 8 mmol/L during the first 24 hours. An overly rapid increase in serum sodium concentration may produce central pontine demyelination |
|
|
Central pontine demyelination
- definition - pathophysiology - symptoms - prognosis |
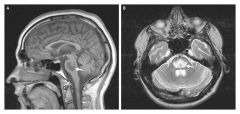
Central pontine myelinolysis- severe damage to the myelin sheath of nerve cells in the brainstem, more precisely in the pons-- usually from an iatrogenic etiology.
- overly rapid correction of serum sodium leads to flowing of water out of cells into the ECF. - symptoms - depends on which part of the brain is involves, but disturbed conciousness, gait changes, seizures, and decreased or absent respiratory function can occur. Also acute para or quadraparesis, dysphagia, dysarthria, diplopia, LOC are common. The patient may experience locked-in syndrome - Prognosis is poor. Most survive but of these patients - 1/3 recover, 1/3 are disabled but can live independently, and 1/3 are severely disabled |
|
|
Hypernatremia
1. definition 2. relation to water volume |
1. plasma Na+ > 145 mmol/L
2. Excess sodium in relation to water, can result from water loss or sodium infusion |
|
|
Hypovolemic hypernatremia
|
- sodium stores are depleted but there is more water loss than sodium loss
- renal loss - from diuretics, osmotic diuresis (most commonly due to glycosuria in diabetics), renal failure - extra renal loss- from diarrhea, diaphoresis, respiratory losses |
|
|
Isovolemic hypernatremia
|
- sodium stores are normal, water is lost
- diabetes insipidus (dec ADH) - insensible respiratory (tachypnea) |
|
|
Hypervolemic hypernatremia
|
- sodium excess-- occurs infrequently
- iatrogenic - most common cause of hypervolemic hypernatremia (e.g. large amount of parenteral NaHCO3, TPN) - exogenous glucocorticoids - Cushing's syndrome - saltwater drowning - primary hyperaldosteronism |
|
|
What are most cases of hypernatremia caused by?
|
- nonrenal water loss- insensible losses, GI tract (diarrhea)
- renal loss- osmotic diuresis (glucosuria), and DI |
|
|
What can happen if you correct hypernatremia too quickly?
|
Cerebral edema can occur because relative to the brain, the blood will have a higher tonicity, which will lead water to flow across the blood brain barrier into the brain and shift into cells.
- the proper rate of correction should NOT exceed 12 mEq/L/day (should be < 8 mEq/L in the first 24 hours) |
|
|
Clinical features of hypernatremia
- secondary to what? - what might cause the sx to be more severe? |
- neurologic sx predominate - AMS, restlessness, weakness, focal neurologic deficits, can lead to confusion, seizures and coma. Tissue and mucous membranes are dry and salivation decreases.
- secondary to osmotic effects on the brain - water shifts out of brain cells, leaving them dehydrated - symptoms may be more prominent and severe when sodium levels increase more rapidly |
|
|
Diagnosis of hypernatremia
|
1. urine volume should be low if the kidneys are responding appropriately
2. Urine osmolality should be > 800 mOsm/kg 3. Desmopressin (synthetic ADH) should be given to differentiate central from peripheral DI if this is suspected |
|
|
Treatment of hypernatremia
1. hypovolemic hypernatremia 2. Isovolemic hypernatremia 3. Hypervolemic hypernatremia |
1. give isotonic NaCl to restore hemodynamic. Correction of hypernatremia can wait until the patient is hemodynamically stable, then replace the free water deficit
2. patients with diabetes insipidus require vasopressin. Prescribe oral fluids or if the patient cannot drink then give D5W 3. - give diuretics (furosemide) and D5W to remove the excess sodium. Dialyze patients with renal failure |
|
|
How do you calculate a free water deficit?
|
Water deficit = (TBW (1- actual Na+))/ desired Na+
|
|
|
What factors contribute to calcium levels?
|
1. Albumin
2. pH 3. hormonal regulation - PTH, calitonin and Vitamin D 4. intake 5. absorption - activated vitamin D |
|
|
How does albumin level affect calcium levels?
|
- Calcium in the plasma exists in two forms: protein-bound and free-ionized
- Protein-bond calcium is bound to albumin, and most calcium ions exist in this state. So therefore the total calcium concentration fluctuates with albumin concentration |
|
|
How does pH affects calcium levels?
|
Changes in pH alter the ratio of calcium binding. An increase in pH results in increased calcium binding to albumin. Therefore in alkalemic states (especially acute respiratory alkalosis), total calcium is normal, but ionized calcium is low and the patient frequent manifests with signs and symptoms of hypocalcemia (tingling, numbness, cramping etc)
|
|
|
How does PTH affect Calcium levels?
|
inc PTH leads to inc plasma Ca2+ and dec plasma phosphate by increasing bone resorption, increasing calcium reabsorption in the kidney and decreasing potassium reabsorption in the kidneys, and increased activated vitamin D levels leading to increased absorption of Calcium from the gut
|
|
|
How does calcitonin affect Ca2+ levels?
|
inc calcitonin leads to dec Ca2+ and dec phosphate by decreasing bone resorption, decreasing Ca2+ reabsorption and increasing phosphate reabsorption. There is decreased absorption of Ca2+ from the gut
|
|
|
How does Vitamin D affect Calcium levels
|
increased Vitamin D leads to increased Calcium and increased phosphate levels by increasing bone resorption, increasing Calcium reaborption in the kidneys and decreasing phosphate reabsorption in the kidneys. In the gut, there increase Ca2+ absorption and phosphate reabsorption
|
|
|
Causes of hypocalcemia
|
1. hypoparathyroidism - most common cause- usually due to surgery on the thyroid gland with damage to the nearby parathyroid glands
2. Acute pancreatitis - deposition of calcium causes lower levels -- saponification seen in fat necrosis 3. renal insufficiency - mainly due to decreased production of 1,25 dihydroxy vitamin D 4. hyperphosphatemia- PO34- precipitates with Ca2+ resultin in calcium phosphate deposition 5. Pseudohypoparathyroidism - autosomal recessive disease causing congenital end-organ resistance to PTH0-- PTH levels are actually high. Also characterized by MR and short metacarpal bones 6. Hypomagnesemia- results in decreased PTH secretion 7. vitamin D deficiency 8. malabsorption- short bowel syndrome 9. Blood transfusion- with citrated blood- calcium binds to citrate 10. osteoblastic mets 11. hypoalbuminemia- but ionized level is normal so it is clinically irrelevant 12. DiGeorge syndreom- deletion on chromosome 22. Absence of thymic shadow on CXR |
|
|
Clinical features of hypocalcemia
|
1. Asx
2. Rickets and osteomalacia 3. Increased neuromuscular irritability a. numbness/tingling- circumoral in fingers, in toes b. tetany- hyperactive DTRs, Cvostek's sign- cheek tapping, Trosseau's sign- BP cuff inflation leads to carpal spasm c. grand mal seizures 4. basal ganglia calcifications 5. cardiac manifestations - arrhthmias, prolonged QT interval |
|
|
Diagnosis of hypocalcemia
|
1. Labs: BUN, Cr, Mg2+, albumin, and ionized calcium. Amylase, lipase and LFTs may also be warranted
2. Serum PO34- - high in renal insufficiency (cannot get rid of it) and hypoparathyroidism, low in primary vitamin D deficiency 3. PTH - low in hypoparathyroidism, elevated in vitamin D deficiency, very high in pseudohypoparathryoidism |
|
|
Treatment of hypocalcemia
|
1. If symptomatic, provide emergency treatment with calcium gluconate
2. For long term management, use oral calcium supplements (calcium carbonate) and vitamin D 3. For PTH deficiency- Vitamin D (calcitriol) plus high oral calcium intake. Thiazide diuretics - lower urinary calcium and prevent urolithiasis 4. correct hypomagnesemia- it is very difficult to correct ca2+ level if you don't correct mag first |
|
|
Causes of hypercalcemia
|
1. Endocrinopathies- hyperparathyroidism, renal failure (usually results in hypocalcemia but can cause secondary hyperparathyroidism), Pagets (osteoclastic reabsorption), acromegaly, addison's disease
2. malignancies- mets- bone mets result in bone destruction due to osteoclastic activity. Most tumors that metastasize to the ones cause both osteolytic and osteoblastic activities (prostate is mainly osteoplastic, while kidney Ca is mostly osteolytic). Multiple myeloma- lysis of tumor cells, release of osteoclast-activating factor by myeloma cells. tumors that release PTH-like hormone (lung ca) 3. pharmacologic- vit D intoxication, milk-alkali syndrome, thiazide diuretics, lithium (inc PTH in some pts), 4. Other - sarcoidosis- increased GI absorption, familial hypocalciuric hypercalcemia |
|
|
Milk-alkali syndrome
|
-repeated ingestion of calcium and absorbable alkali (calcium carbonate, milk, sodium bicarbonate)
- hypercalcemia, alkalosis, renal impairment (e.g. eating too many tums) |
|
|
Clinical features of hypercalcemia
|
Stones, Bones, Groans, and Psychiatric overtones
1. Stones- nephrolithiasis and nephrocalcinosis 2. Bones- Bone aches and pains, osteitis fibrosa cystica ("brown tumors") predisposes to pathologic fractures 3. Groans- muscle pain and weakness, pancreatitis, peptic ulcer disease, gout, constipation 4. psychiatric overtones- depression, fatigue, anorexia, sleep disturbances, anxiety and lethargy 5. Other findings- polydipsia, polyuria, HTN, weight loss, short QT interval or patients may be asx |
|
|
Diagnosis of hypercalcemia
|
1. Same as hypocalcemia (Labs: BUN, Cr, Mg2+, albumin, and ionized calcium, PTH, phosphate)
2. Radioimmunoassay of PTH - elevated in primary hyperparathyroidism, low in occult malignancy 3. Radioimmunoassay of PTHrP- elevated in malignancy 4. Bone scan or bone survey to identify lytic lesions 5. urinary cAMP- markedly elevated in primary hyperparathyroidism |
|
|
Treatment of hypercalcemia
|
1. Increase urinary excretion - IV fluids (NS) - first step in management, diuretics (furosemide) - inhibit Calcium reabsorption
2. Inhibit bone resorption in patients with osteoclastic disease (e.g. malignancy)- bisphosphonates (pamidronate) and calcitonin 3. Give glucocorticoids if vit D related mechanism (intoxication, granulomatous disorders) and multiple myeloma are the causes 4. use hemodialysis for renal failure patients 5. phosphate is effective but incurs risk of metastatic calcification |
|
|
Potassium Metabolism
1. normal K+ levels 2. location in the body 3. hypokalemia 4. hyperkalemia 5. Potassium secretion |
1. normal levels- 3.5- 5.0 mEq/L
2. Location in the body- most of the body's potassium (98%) is intracellular 3. Hypokalemia- alkalosis and insulin administration can cause hypokalemia because they shift potassium into cells 4. Hyperkalemia- acidosis and anything resulting in cell lysis can increase serum K+ (both force K+ out of cells and into the ECF) 5. Potassium secretion - most of the excretion of potassium occurs through the kidneys (80%); the remainder occurs via the GI tract. Aldosterone plays an important role in renal K+ secretion. Kicks K+ out. |
|
|
Causes of hypokalemia
1. GI causes 2. Renal causes 3. Other causes |
1. GI- vomiting and NG suction (volume depletion and metabolic alkalosis also result), diarrhea, laxatives and enemas, intestinal fistulae, decreased potassium absorption in intestinal disorders
2. Renal losses- diuretics (all except Spironolactone, Amiloride and Triamterene (SAT), renal tubular or parenchymal disease, primary and secondary hyperaldosteronism, excessive glucocorticoids, magnesium deficiency, Bartter's syndrome (see separate card) 3. other causes - insufficient dietary intake, insulin admin, certain abx (esp Bactrim and amphoteracin B), profuse sweating, epi (b2) agonists- hypokalemia occurs in 50% to 60% of trauma patients, perhaps due to this increase in epi levels, albuterol can also cause K+ to shift into cells |
|
|
how can urine K+ levels help you determine whether the source of hypokalemia is due to renal or GI losses?
|
If the cause of hypokalemia is due to gastric losses then the kidneys should respond appropriately and retain K+, leading to lower urine K+ levels (<20 mEq/L), whereas if the cause is renal then urinary K+ levels will be > 20 mEq/L
|
|
|
What is a common cause of both non-anion gap metabolic alkalosis and hypokalemia?
|
diarrhea. The alkalosis worsens the hypokalemia by causing K+ to shift into cells
|
|
|
How does the presence or absence of HTN help you differentiate between different causes of hypokalemia?
|
If the cause is hyperaldosteronism, then HTN will likely be present, whereas if GI or renal causes are present then there will NOT be HTN
|
|
|
What are the most dangerous complications of hypokalemia?
|
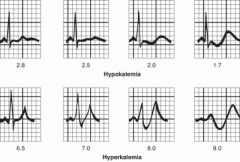
Arrhythmias- on ECG you may see T wave flattening at first. If it is severe you may see T-wave inversion and appearance of U-waves
|
|
|
What electrolyte should you always monitor in patients taking digoxin and why?
|
Potassium as hypokalemia predisposes to digoxin toxicity because of the mechanism of the drug (inactivation of the Na+/K+ ATPase pump, which leads to increase intracellular calcium concentration and thus increased contractility)
|
|
|
Clinical features of hypokalemia?
|
1. Arrhythmias- prolongs cardiac conduction
2. muscular weakness, fatigue, paralysis and muscle cramps 3. decreased DTRs 4. paralytic ileus 5. polyuria and polydipsia 6. n/v 7. exacerbates digoxin toxicity 8. flattening of T waves on ECG and U waves appear if severe. |
|
|
Treatment of hypokalemia
|
1. Identify and treat the underlying cause
2. Discontinue any medications that can aggravate hypokalemia 3. Oral KCl is the preferred (safest) method of replacement and is appropriate in most cases. Always retest K+ levels after administration. - using 10mEq of KCl increases K+ levels by 0.1 mEq/L. It comes in fast-acting and slow-acting forms 4. IV KCl can be given if hypokalemia is severe (<2.5) or if the patient has arrhythmias secondary to hypokalemia - give slowly to avoid hyperkalemia, monitor K+ concentration and cardiac rhythm. Max infusion rate in peripheral IV is 10mEq/hr and 20 mEq/hr in a central line. May add 1% lidocaine to bag to decrease pain cuz it burns going in 5. as with ca2+, it is difficult to correct K+ level if Mag levels are not corrected first |
|
|
Causes of hyperkalemia
1. increased total body potassium 2. redistribution 3. pseudohyperkalemia (spurious) |
1. Increased total body K+ - renal failure, Addison's disease, K+ sparing diuretics (e.g. spironolactone), hyporeninemic hypoaldosteronism, ACE inhibitors, iatrogenic overdose, blood transfusion
2. Redistribution- translocation of K+ from intracellular to extracellular space- acidosis, tissue/cell breakdown-- rhabdo, hemolysis, burns, GI bleeding, Insulin deficiency, rapid admin of a beta blocker 3. pseudohyperkalemia- artifically elevated plasma K+ conc due to K+ moving out of cells immediately before or after venipuncture (prolonged tourniquet use), if sample is not processed quickly some RBCs may lyse. Leukocytosis and thrombocytosis may also contribute. Repeat test if this is suspected. |
|
|
Clinical features of hyperkalemia
|
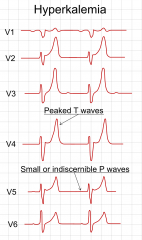
1. Arrhythmias- the most important effect of hyperkalemia is on the heart. Check an ECG immedicately. With increasing potassium, ECG changes progress through tall, peaked T waves, QRS widening, PR prolongation, loss of p waves and finally a sine wave pattern (Vfib)
2. muscle weakness and (rarely) flaccid paralysis 3. decreased DTRs 4. respiratory failure 5. n/v, intestinal colic, diarrhea |
|
|
Treatment of hyperkalemia
|
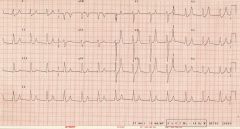
1. GIVE IV CALCIUM to stabilize the resting membrane potential of the myocardial membrane and decrease excitability
2. Use caution in giving calcium to patients on digoxin (hypercalcemia predisposes to digoxin toxicity) 3. Shift potassium into the intracellular department by giving - glucose and insulin (glucose alone will stimulate insulin, but exogenous insulin is more rapid), give both to prevent hypoglycemia. Sodium bicarbonate- increases pH level, which shifts K+ into cells. This is an emergency measure for severe hyperkalemia. 4. Remove potassium from the body- a. kayexalate- GI potassium exchange resin absorbs K+ in colon and thus prevents reabsorption (passed in stool), b. hemodialysis- most rapid and effective way of lowering plasma K+, reserved for intractable hyperkalemia and for those in renal failure. c. diuretics (furosemide) |
|
|
Magnesium Metabolism
1. Normal range 2. location in the body 3. influences on mag excretion 4. magnesium absorption and balance |
1. normal mg2+ levels : 1.8 - 2.5 mg/dL
2. location in the body- most of the mag is in the bones (2/3) and the remainder is intracellular (1/3). Only 1% of mag is extracellular 3. many hormones can alter urinary mag excretion (e.g. insulin/glucagon, PTH, calcitonin, ADH and steroids) 4. mag absorption and balance- about 30-40% of dietary magnesium is absorbed in the GI tract, but this percentage increases when mag levels are low. The kidney has a great capacity to reabsorb magnesium and is the major regulator of mag balance |
|
|
Causes of hypomagnesemia
|
1. GI causes- malabsorption, steatorrheic states (most common cause), prolonged fasting, fistulas, patients receiving TPN without mag
2. Alcoholism (common cause) 3. renal causes- SIADH, diuretics, Bartter's syndrome, drugs (gentamycin, amphoteracin B, cisplatin), renal transplantation 4. other causes: postparathyroidectomy, DKA, thyrotoxicosis, lactation, burns, pancreatitis, cisplatin |
|
|
Bartter's syndrome
|
- chronic volume depletion secondary to AR defect in salt reabsorption in the thick ascending loop of Henle leads to hyperplasia of the juxtaglomerular apparatus, which leads to increased renin levels and secondary aldosterone elevations
|
|
|
Clinical features of hypomagnesemia
1. CNS and muscle 2. effect on calcium levels 3. effect on potassium levels 4. ECG changes |
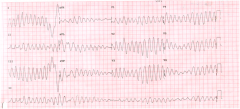
1. marked neuromuscular and CNS hyperirritability
- muscle twitching, weakness and tremors - hyperreflexia, seizures - AMS 2. Effect on calcium levels - coexisting hypocalcemia is common because of decreased PTH lebel and bone resistance to PTH when Mg2+ is low. 3. Effect on potassium levels - co-existing hypokalemia is up to 50% of cases. In muscle and myocardium, when either intracellular mag or K+ decreases, a corresponging decrease in the other cation takes place 4. ECG changes- prolonged QT interval, T wave flattening, and ultimately torsade de pointes |
|
|
Treatment of hypomagnesemia
|
1. For mild hypomag- oral Mg2+ (e.g. magnesium oxide)
2. For severe hypomag- parenteral Mg2+ (e.g. magnesium sulfate) |
|
|
Hypermagnesemia causes
|
1. Renal failure (most common cause)
2. early-stage burns, massive trauma, or surgical stress, severe ECF deficit, severe acidosis 3. Excessive intake of magnesium containing laxatives or antacides combined with renal insufficiency 4. adrenal insufficiency 5. rhabdomyolysis 6. Iatrogenic- in the OB setting in women with pre-eclampsia or eclampsia being treated with mag sulfate |
|
|
Clinical features of hypermagnesemia
|
1. nausea, weakness
2. facial paresthesias 3. progressive loss of DTRs (classically the first sign) 4. ECG changes resemble those seen with hyperkalemia ( increased PR interval, widened WRS and elevated T waves) 5. somnolence leading to coma and muscular paralysis occur late 6. death is usually caused be respiratory failure or cardiac arrest |
|
|
Phosphate metabolism
1. normal phos levels 2. location in the body 3. influence on phosphate absorption 4. phosphate excretion and balance |
1. normal phos levels - 3.0 - 4.5 mg/dL
2. location in the body- most is in the bones (85%), the remainder is in ICF in soft tissues (15%) and very small amount is in ECF 3. Influence on absorption- vitamin D controls phos absorption in the GI tract 4. phosphate excretion and balance- PTH controls excretion in the kidney - PTH increases renal phosphorus excretion by inhibiting reabsorption. The function of the kidney in maintaining phosphate balance is very important |
|
|
Causes of hypophosphatemia
|
1. decreased intestinal absorption due to alcohol abuse, vitamin D deficiency, malabsorption of phosphate, excessive use of phosphate-binding antacids, hyperalimentation (TPN), and/or starvation
2. Increased renal excretion - excess PTH states (vit D deficiency, hyperparathyroidism) - hyperglycemia (glycosuria), oncogenic osteomalacia, ATN, renal tubular acidosis - hypokalemia or hypomagnesemia 3. other causes- respiratory acidosis, anabolic steroids, severe hyperthermia, DKA, hungry bone syndrome (deposition of bone material after parathyroidectomy) |
|
|
Clinical features of hypophosphatemia
|
1. none - if hypophosphatemia is mild
2. any of the following if severe: neuro: encephalopathy, confusion, seizures, numbness, parathesias. MSK- muscular weakness, myalgias, bone pain, rickets/osteomalacia. Heme- hemolysis, RBC dysfunction, WBC dysfunction, platelet dysfunction, Cardiac- cardiomyopathy and myocardial depression secondary to low ATP levels, may lead to cardiac arrest. Rhabdo, anorexia, difficulty in ventilator weaning |
|
|
Treatment of hypophosphatemia
|
1. If mild ( > 1mg/dL), oral supplementation: Neutra-phos capsules, K-phos tablets, milk (excellent source of phosphate)
2. if severe/symptomatic or if the patient is NPO- parenteral supplementation |
|
|
What are the most common causes of hypophosphatemia? (2)
|
Alcohol abuse and DKA
|
|
|
Hyperphosphatemia causes
|
1. decreased renal excretion of PO43- due to renal insufficiency (most common cause), bisphosphonates, hypoparathyroidism, vitamin D intoxication, and or tumor calcinosis
2. increased phosphate admin (repletion or phos containing enemas) 3. Rhabdo, cell lysis, or acidosis (releases phos into the ECF) |
|
|
Clinical features of hyperphosphatemia
|
1. this results in metastatic calcification and soft-tissue calcifications: a calcium-phosphorus product (serum calcium x serum phos) > 70 indicates the calcification is likely to occur
2. The associated hypocalcemia can lead to neuro changes (tetany, neuromuscular irritability) |
|
|
Treatment of hyperphosphatemia
|
1. phosphate-binding, antacids containing aluminum hydroxide or carbonate (bind phosphate in the bowel and prevent its absorption)
2. hemodialysis (if patient has renal failure) |
|
|
Metabolic acidosis- general characteristics
|
- decrease in blood pH and a decreased plasma bicarbonate concentration. The goal is to identify the underlying condition
- calculate the anion gap by = [Na+] - ([Cl-] + [HCO3-]) - positive ion minus the negative ones - reflects ions present in the serum but unmeasured (i.e. proteins, phosphates, organic acids, sulfates) - normal values are 5-15 mEq/L but this varies to some extent |
|
|
Effects of acidosis
|
- right shift in oxy-hemoglobin dissociation curves that diminishes the affinity of hemoglobin for oxygen (so increases oxygen delivery to the tissues)
- depresses CNS - decreases pulmonary blood flow - arrythmias - impairs myocardial function - hyperkalemia |
|
|
Effects of alkalosis
|
- decreases cerebral blood flow
- left shift in oxygen-hemoglobin dissociation curve that increases the affinity of Hgb for o2 and thus decreases oxygen delivery to the tissues - arrhythmias - tetany - seizures |
|
|
How do lactate levels affect bicarb levels?
|
an increase in lactate levels results in a decrease in bicarb levels due to buffering in ECF. The kidneys then respond by increasing HCO3- reabsorption to maintain pH
|
|
|
Pathophysiology of metabolic acidosis
|
- when fixed acid (lactate) is added, the H+ from the fixed acid is buffered by the bicarb system. CO2 is formed and removed by the lungs. H+ + HCO3- <--> H2CO3 <--> H20 + CO2. HCO3- levels decrease in the ECF, therefore the kidneys reabsorb more to maintain the pH
- 3 situations can arise: a) the change in AG equals the change in HCO3- - simple metabolic acidosis-- the addition of acid causes AG to increase proportionally b) the change in AG is less than the change in HCO3-- normal AG PLUS high levels of AG acidosis- If after the addition of acid, the HCO3- is lower than the calculate prediction, the you must have started with a lower HCO3- - The change in AG is greater than the change in HCO3- resulting in a metabolic alkalosis PLUS high AG acidosis- when you have a high AG, the acid is buffered by HCO3- so HCO3- decreases. If HCO3- does not decrease, it means you started with a high HCO3- |
|
|
Causes of increased anion-gap acidosis
|
MUDPILES- Methanol, Uremia (chronic renal failure), DKA, Propylene glycol, Infection, Iron, INH, inborn errors of metabolism, Lactic acidosis, Ethylene glycol (antifreeze), Salicylates
- ketoacidosis - DM, prolonged starvation, prolonged EtOH abuse - lactic acidosis- low tissue perfusion, shock states, excessive expenditure of energy (e.g. seizures) - renal failure- decreased NH4+ excretion- increases net acid - decreased excretion of organic anions, sulfate and phosphate inc the AG - intoxication - aspirin, methanol, ethylene glycol |
|
|
Causes of non-anion gap metabolic acidosis (hyperchloremic metabolic acidosis)
|
- the low HCO3- is associated with a high Cl-, so the AG remains normal
- renal loss of bicarbonate - proximal tubular acidosis (characterized by decreased HCO3- reabsorption. causes include MM, cystinosis, and Wilson's. Distal tubular acidosis- characterized by inability to make HCO3- causes include SLe, Sjogrens, and taking amphoteracin B, carbonic anhydrase inhibition (e.g. taking the diuretic acetazolamide) - GI loss of HCO3- : diarrhea- HCO3- loss in diarrhea (most common cause of non-AG acidosis), pancreatic fistulas (pancreatic secretions contain high levels of HCO3- ), small bowel fistulas, ureterosigmoidostomy - colon secretes HCO3- in the urine in exchange for Cl- |
|
|
Clinical features of metabolic acidosis
|
1. Hyperventilation (deep rhythmic breathing) also known as Kussmaul's respiration in order to compensate for the metabolic acidosis. This a cardinal feature and is usually seen in severe met acidosis (pH < 7.20). It is less prominent when the acidosis is chronic
2. decreased cardiac output and decreased tissue perfusion - occurs when severe met acidosis ( pH < 7.2). Acidosis diminishes tissue response to catecholamines. This can lead to an undesirable chain of events --> poor tissue perfusion --> lactic acidosis --> decreased C.O. --> hypotension --> further decrease in tissue perfusion |
|
|
Diagnosis of metabolic acidosis
|
1. History is important
2. Calculate the AG 3. Winter's formula to calculate the expected PaCO2 = 1.5 (measured HCO3-) + 8 +/- 2. - This predicts the expected respiratory compensation (PaCO2 level) to metabolic acidosis. If the PaCO2 level does not fall within the acceptable range, the the patient has another primary acid-base disorder - if the PaCO2 is within the predicted range, then the patient has met acidosis with appropriate response- secondary hypocapnia - If the actual PaCO2 is higher the expected PaCO2 the patient has met acidosis and a resp acidosis. This is a serious finding because the failure of compensation can be a sign of impending respiratory failure (the classic example is an asthmatic child who has a PacO2 that goes from abnormal to normal with no treatment. This is a bad sign, and it probably means the child needs emergent intubation. d. the actual PaCO2 is lower than the expected PaCO2, the patient has metabolic acidosis with a respiratory alkalosis |
|
|
Treatment of metabolic acidosis
|
1. treatment depends on the underlying cause
2. sodium bicarb is sometimes needed (especially for normal AG acidosis). In correcting metabolic acidosis (correct severe acidosis to a pH of 7.20), realize that this HCO3- takes 24 hours to get to the brain. During this time, hyperventilation continues. Therefore PaCO2 remains low while HCO3- is increasing which is a dangerous combination ([H+] = 24 [PaCO2/HCO3-] - mechanical ventilation may be required if the pt is fatigued from prolonged hyperventilation, especially in DKA |
|
|
Metabolic alkalosis- general characteristics
|
General characteristics - metabolic alkalosis is characterized by an increased blood pH and plasma HCO3-
- uncomplicated metabolic alkalosis is typically transient, because the kidneys can normally excrete excess HCO3- |
|
|
What 2 events should be considered in metabolic alkalosis
|
1. Event that initiates the metabolic alkalosis (loss of H+ via gastric drainage, vomiting and so on) or increased HCO3- conc due to ECF volume contraction
2. mechanism that maintains the metabolic alkalosis due to the kidney's inability to excrete excess HCO3- |
|
|
Causes of metabolic alkalosis
1. saline-sensitive 2. saline-resistant |
1. Saline-sensitive metabolic alkalosis (urine chloride < 10 mEq/L) characterized by ECF contraction and hypokalemia
a. vomiting and NG suction - when the patient loses HCl-, gastric HCO3- generation occurs which causes an alkalosis b. diuretics- these decrease the ECF volume. Body HCO3- content remains normal, but plasma HCO3- increases because of ECF contraction c. villous adenoma or the colon, diarrhea with a high chloride content 2. saline--resistant metabolic alkalosis (urine chloride > 20 mEq/L)- characterized by ECF expansion and hypertension (due to increased mineralocorticoids). a. most are secondary to adrenal disorders (primary hyperaldosteronism). Increased levels of mineralocorticoid secretion lead to increased tubular reabsorption of Na+ and HCO3- and an excessive loss of Cl- in the urine b. other causes include cushing's syndrome, severe K+ deficiency, Bartter's syndrome, and diuretic abuse |
|
|
Clinical features of metabolic alkalosis
|
1. There are no characteristic signs or symptoms
2. the patient's medical history is most helpful (look for vomiting, gastric drainage, diuretic therapy etc) |
|
|
Diagnosis of metabolic alkalosis
|
1. Elevated HCO3- level, elevated pH level
2. Hypokalemia is common 3. PaCO2 is elevated as a compensatory mechanism due to hypoventilation. It is rare for a compensatory increase in PaCO2 to exceed 50-55 mm Hg as the respiratory rate to achieve this would drop the PaO2. A higher value implies a superimposed respiratory acidosis 2. the urine chloride level is very important in distinguishing saline-sensitive from saline-resistant types |
|
|
Treatment of metabolic alkalosis
|
1. Treat the underlying disorder that caused the metabolic alkalosis
2. Normal saline plus potassium will restore the ECF volume if the patient is volume contracted 3. Address the underlying cause (or prescribe spironolactone- aldo inhibitor) if the patient is volume expanded |
|
|
Respiratory Acidosis- general characteristics
|
1. Defined as a reduced blood pH and PaCO2 > 40 mmHg
2. Renal compensation (increased reabsorption of HCO3-) begins within 12-24 hours and takes 5 days or so to complete - acute respiratory acidosis - there is an immediate compensatory elevation of HCO3-. There is an increase of 1 mmol/L for every 10 mmHg increase in PaCO2 - chronic respiratory acidosis- renal adaptation occurs and HCO3- increases by 4 mmol/L for every 10 mm Hg in PaCO2. This is generally seen in patients with underlying lung disease, such as COPD * any disorder that reduces CO2 clearance (i.e. inhibits adequate ventilation) can lead to respiratory acidosis * |
|
|
How do you distinguish between metabolic alkalosis with volume contraction and metabolic alkalosis with volume expansion?
|
Urinary chloride concentration. If there is volume expansion then the urine chloride will be high (> 20 mEq/L)
|
|
|
Causes of alveolar hypoventilation
|
1. primary lung disease - for example, COPD, airway obstruction
2. neuromuscular disease- e.g. myastenia gravis 3. CNS malfunction - injury to the brainstem 4. Drug- induced hypoventilation (e.g. morphine, anesthetics or sedatives). Narcotic overdose in post-op patients is a possibility (look for pinpoint pupils) 5. Respiratory muscle fatigue |
|
|
Clinical features of respiratory acidosis
|
1. Somnolence, confusion and myclonus (brief involuntary muscle twitching) with asterixis
2. headaches, confusion, and papilledema are signs of acute CO2 retention |
|
|
Treatment of respiratory acidosis
|
1. verify airway patency
2. If PaO2 is low (< 60 mmHg), initiate supplemental oxygen. CAUTION- in some patients who are CO2 retainers (COPD patients)- oxygen can exacerbate the respiratory acidosis by removing the hypoxemic stimulus to breathe 3. Correct reversible causes 4. any measure to improve alveolar ventilation -aggressive pulmonary toilet, correct reversible pulm disease (e.g. tx pneumonia), remove obstruction, naloxone for opiate OD, bronchodilators 5. intubation and mech vent may be necessary to relieve the acidemia and hypoxia in the following situations: severe acidosis, PaCO2 > 60 or inability to increase PaO2 with supplemental oxygen, if the patient has decreasing mental status, impending respiratory fatigue signaled by labs or prolonged labored breathing |
|
|
Respiratory Alkalosis - general characteristics
|
1. Increased blood pH and decreased PaCO2
2. in order to maintain blood pH within the normal range, HCO3- must decrease, so renal compensation occurs. However, this does NOT occur acutely, but rather over the course of several hours - acutely for each 10 mmHg decrease in PaCO2, plasma HCO3- decreases by 2 mEq/L and blood pH increases by 0.08 mEq/L - chronically for each 10 mmHg decrease in PaCO2, plasma HCO3- decreases by 5-6 mEq/L and blood pH decreases by 0.02 mEq/L |
|
|
Causes of respiratory alkalosis
|
Alveolar hyperventilation
1. anxiety 2. pulmonary embolus, pneumonia, asthma 3. sepsis 4. hypoxia - inc RR 5. mech vent 6. pregnancy - inc serum progesterone levels cause hyperventilation 7. liver disease (cirrhosis) 8. medication (salicylate toxicity) 9. hyperventilation syndrome |
|
|
Clinical features of respiratory alkalosis
|
1. symptoms are mostly related to decreased cerebral blood flow (vasocontriction) -- LH, dizziness, paresthesia, and perioral numbness
2. tetany (indistinguishable from hypocalcemia) 3. arrhythmias (in severe cases) |
|
|
Treatment of respiratory alkalosis
|
1. correct the underlying cause
2. sometimes this does not need to be treated (e.g. pregnancy) 3. an inhaled mixture containing CO2 or breathing into a paper bag may be useful to slow down ventilation |
|
|
How does respiratory acidosis lead to generalized CNS depression?
|
increased PaCO2--> increased cerebral blood flow --> increased CSF pressure--> generalized CNS depression
|
|
|
What are the two primary determinants of PaCO2?
|
respiratory rate and tidal volume
|

