![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
228 Cards in this Set
- Front
- Back
- 3rd side (hint)
Fill in
|
answers
|
|
|
fill in
|
answers
|
|
|
fill in
|
answers
|
|
|
|
Why is the left kidney taken during living donor transplantation?
|
Because the left renal vein is longer.
|
|
|
|
What course do the ureters take from the kidney to the urethra?
|
"Water under the bridge"
Ureters pass under uterine artery and ductus deferens (retroperitoneal) |
|
|
|
List the different body fluid compartments and their relative volumes.
|
TBW: 40% nonwater mass, 60% total body water
Total body water: 1/3 ECF, 2/3 ICF ECF: 1/4 plasma, 3/4, interstitial |
|
|
|
Is sodium or potassium high inside the cell?
|
"HIKIN"
High K INtracellular |
|
|
|
What is the 60-40-20 rule?
|
60% total body water
40% ICF 20% ECF |
|
|
|
How can plasma volume be measured?
|
Radiolabeled albumin.
|
|
|
|
How can extracellular volume be measured?
|
Inulin
|
|
|
|
What is the typical osmolarity?
|
290 mOsm
|
|
|
|
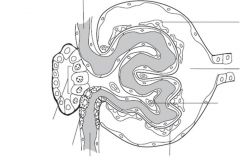
What does the glomerular filtration barrier consist of?
|
1. Fenestrated capillary endothelium
2. Basement membrane fused with heparin sulfate (negative charge barrier). 3. Epithelial layer consisting of podocyte foot processes (pedicles). |
|
|
|
What happens to the charge barrier in nephrotic syndrome?
|
Charge barrier is lost resulting in albuminuria, hypoproteinemia, generalized edema, and hyperlipidemia.
|
|
|
|
What equation describes the renal clearance?
|
Cx=(Ux)(V)/Px
Cx is clearance of drug x Ux is urine concentration of drug x Px is plasma concentration of drug x V is urine flow rate |
|
|
|
What are the units for clearance of a compound?
|
mL/min
|
|
|
|
Relating the clearance of a compound to the glomerular filtration rate can tell you what? Provide examples.
|
Whether the compound is reabsorbed or secreted.
Cx<GFR, reabsorption Cx>GFR, secretion Cx=GFR, no net secretion or absorption |
|
|
|
What compound can be used to calculate the GFR and why?
|
By monitoring inulin because it is freely filtered, but neither secreted nor reabsorbed.
|
|
|
|
Creatinine clearance can be used to estimate the GFR. What are the limitation to its use in this regard?
|
Creatinine is moderately secreted by the renal tubules, so it slightly overestimates GFR.
|
|
|
|
What equation can be used to calculate GFR based upon inulin clearance?
|
GFR=Cinulin=(Ui)(V)/Pi
|
|
|
|
What can be used to estimate the effective renal plasma flow?
|
Clearance of PAH (para-aminohippurate) because it is both filtered and excreted.
|
|
|
|
How closely does the effective renal plasma flow estimate the true renal plasma flow?
|
It underestimates RPF by approximately 10%
|
|
|
|
How can one calculate the filtration fraction?
|
FF=GFR/RPF
GFR estimated with creatinine clearance RPF estimated with PAH clearance |
|
|
|
What is the normal filtration fraction?
|
20%
|
|
|
|
What is the filtered load calculated as?
|
GFR x plasma concentration
|
|
|
|
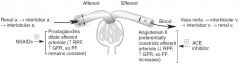
What compounds dilate afferent arterioles, leading to increased RPF and GFR?
|
Prostaglandins (inhibited by NSAIDs).
|
|
|
|
When prostaglandins are produced, what happens to the filtration fraction?
|
It remains constant since both RPF and GFR increase proportionally.
|
|
|
|
What compound constricts efferent arterioles, leading to decreased RPF and increased GFR?
|
Angiotensin II (inhibited by ACE inhibitors).
|
|
|
|
When angiotensin II is produced, what happens to the filtration fraction?
|
It increases because RPF decreases and GFR increases.
|
|
|
|
What effect does increased plasma protein concentration have on the RPF, GFR, and FF?
|
RPF unchanged
GFR decreases FF decreases |
|
|
|
What effect does decreased plasma protein concentration have on RPF, GFR, and FF?
|
RPF is unchanged
GFR is increased FF is increased |
|
|
|
What effect does constriction of the ureter have on RPF, GFR, and FF?
|
RPF is unchanged
GFR is decreased FF is decreased |
|
|
|
What is the definition of free water clearance (CH2O)?
|
The volume of blood plasma that is cleared of solute-free water per unit time.
|
|
|
|
How can free water clearance be calculated?
|
CH2O=V-Cosm
V=urine flow rate Cosm=UosmV/Posm |
|
|
|
What effect does anti-diuretic hormone (ADH) have on free water clearance?
|
CH2O<0
retention of free water |
|
|
|
What happens to free water clearance in the absence of ADH?
|
CH2O>0
excretion of free water |
|
|
|
What effect do loop diuretics have on free water clearance?
|
They create isotonic urine, making CH2O equal to zero.
|
|
|
|
How can the reabsorption rate of a compound be calculated?
|
Reabsorption rate = filtered load - excretion rate
= (GFR x Px) - (V x Ux) |
|
|
study
|
study
|
|
|
|
At a normal plasma level of glucose, what proportion is reabsorbed in the proximal tubule?
|
All of it.
|
|
|
|
At what plasma glucose concentration does glucosuria begin (threshold)?
|
160-200 mg/dL
|
|
|
|
What is significant about plasma glucose concentrations of 350 mg/dL
|
All of the Na+/glucose transporters in the proximal tubule are saturated.
|
|
|
|
What is the clinical significance of glucosuria?
|
It is an important clinical clue of diabetes mellitus.
|
|
|
|
How are amino acids reabsorbed?
|
By sodium-dependent transporters (at least 3) in the proximal tubule.
|
|
|
|
What happens in Hartnup's disease?
|
A deficiency of neutral amino acid (tryptophan) transporter, resulting in pellegra.
|
|
|
study
|
study
|
|
|
study
|
study
|
|
|
study
|
study
|
|
|
study
|
study
|
|
|
|
What happens in the early proximal tubule?
|
Brush border enzymes reabsorb most all glucose and amino acids, and most bicarbonate, sodium, chloride, and water. It generates and secretes ammonia which buffers the H+ secretion.
|
|
|
|
What happens in the thin descending loop of Henle?
|
Passive reabsorption of water due to medullary hypertonicity. This segment is impermeable to sodium.
|
|
|
|
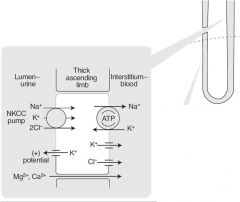
What happens in the thick ascending loop of Henle?
|
Active reabsorption of sodium, potassium, chloride and paracellular reabsorption of magnessium and calcium. This segment is impermeable to water.
|
|
|
|
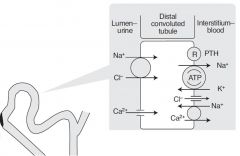
What happens in the distal convoluted tubule?
|
Active reabsorption of sodium and chloride.
|
|
|
|
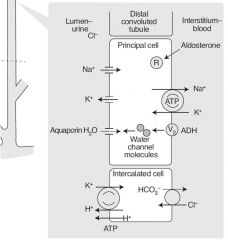
What happens in the collecting tubules?
|
Reabsorption of sodium in exhange for secreting potassium and hydrogen ions. Passive secretion of water by aquaporins.
|
|
|
|
What effect does PTH have on different segments of the nephron?
|
1. Inhibits sodium/phosphate cotransport in the proximal tubule leading to phosphate excretion.
2. Increases calcium/sodium exchange in distal convoluted tubule leading to calcium reabsorption. |
|
|
|
What effect does Angiotensin II have on the nephron?
|
It stimulates sodium/hydrogen exchange in the proximal convoluted tubule leading to sodium and water reabsorption. This permits contraction alkalosis.
|
|
|
|
What effect does aldosterone have on the nephron?
|
It leads to insertion of sodium channels on luminal side of cells in the collecting tubules. This causes reabsorption of sodium and water.
|
|
|
|
What effect does ADH have on the nephron?
|
It acts at V2 receptors, increasing aquaporin channels on the luminal side of the collecting tubules.
|
|
|
|
How do the concentrations of creatinine and inulin change along the proximal tubule?
|
They increase in concentration because of water reabsorption.
|
|
|
|
What happens to the chloride concentration in the proximal tubule?
|
It increases along the proximal 1/3 and then plateaus along the distal 2/3.
|
|
|
|
What drives water reabsorption in the proximal tubule?
|
sodium
|
|
|
|
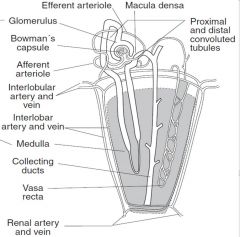
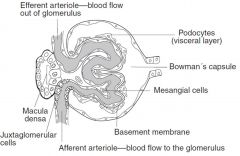
What cells detect changes in blood pressure?
|
Juxtaglomerular cells.
|
|
|
|
What cells detect sodium chloride concentrations in the distal tubule?
|
Macula densa cells.
|
|
|
|
What three factors regulate renin release from juxtaglomerular cells.
|
↓ BP, ↓ sodium delivery, ↑ sympathetic tone (β1 receptors)
|
|
|
|
What is the primary function of renin?
|
It converts angiotensinogen into angiotensin I.
|
|
|
|
Where is angiotensinogen produced?
|
In the liver.
|
|
|
|
Where is angiotensin converting enzyme do and where is it secreted?
|
It is synthesized in the lungs and kidney and It converts angiotensin I to angiotensin II and degrades bradykinin.
|
|
|
|
What are six primary functions of angiotensin II?
|
1. Acts at AT2 receptors on vascular smooth muscle, causing constriction.
2. Constricts efferent arteriole of glomerulus 3. Stimulates aldosterone secretion from the adrenal gland. 4. Stimulates anti-diuretic hormone secretion from the posterior pituitary gland. 5. Increases proximal tubule Na/H activity 6. Stimulates hypothalamus to cause thirst |
|
|
|
Why does angiotensin II not result in reflex bradycardia?
|
It affects baroceptor function limiting reflex bradycardia.
|
|
|
|
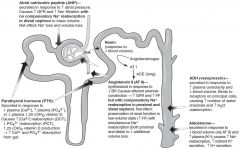
How is the heart tied into the renin-angiotensin-aldosterone system?
|
Atrial natriuretic peptide is released in response to ↑ BP and relaxes vascular smooth muscle via cGMP causing ↑ GFR and ↓ renin.
|
|
|
|
What does antidiuretic hormone respond to?
|
It primarily regulates osmolarity but also responds to low blood volume, which takes precedence over osmolarity.
|
|
|
|
What does aldosterone do?
|
It primarily regulates blood volume.
|
|
|
|
What compounds protect blood volume in low-volume states?
|
Both ADH and aldosterone.
|
|
|
|
What happens following engagement of AT II receptors by angiotensin II?
|
Vasoconstriction of arterioles leading to increased BP.
|
|
|
|
What do juxtaglomerular cells do?
|
They defend GFR via renin-angiotensin-aldosterone system. The secrete renin in response to ↓ renal BP, ↓ Na delivery to distal tubule, and ↑ sympathetic tone.
|
|
|
|
What do macula densa cells do?
|
The sense sodium in the distal convoluted tubule and signal to juxtaglomerular cells.
|
|
|
|
What are the primary endocrine functions of the kidney?
|
Erythropoietin, 1,25-(OH)2 vitamin D, renin, prostaglandins.
|
|
|
|
What signals erythropoietin release from the kidney?
|
hypoxia
|
|
|
|
Describe the role of the kidney in calcium and phosphate metabolism.
|
PTH leads to ↑ calcium and ↓ phosphate reabsorption in the kidney. It also stimulates 1,25 dihydroxy vitamin D production in proximal tubule cells, which promote intestinal absorption of calcium and phosphate.
|
|
|
|
How do prostaglandins synthesized in the kidney function, and what may cause problems with this?
|
Prostaglandins function as paracrine hormones to dilate afferent arterioles. NSAIDs may cause acute renal failure by inhibiting this process.
|
|
|
|
What kidney enzyme is stimulated by PTH to produce 1,25 dihydroxy vitamin D?
|
1α-hydroxylase
|
|
|
study
|
study
|
|
|
|
What is the overall effect of ANP?
|
Causes increased GFR and sodium filtration with no compensatory sodium reabsorption in distal nephron. This leads to sodium and water loss.
|
|
|
|
What three factors stimulate PTH secretion from the posterior pituitary gland?
|
↓ plasma calcium, ↑ plasma phosphate, ↓ plasma 1,25 dihydroxy vitamin D
|
|
|
|
List six conditions that can lead to potassium shift out of cells (hyperkalemia).
|
1. Insulin deficiency (↓ Na/K ATPase)
2. beta-adrenergic antagonists (↓ Na/K ATPase) 3. Acidosis, severe excersize (K/H exchanger) 4. Hyperosmolarity 5. Digitalis (blocks Na/K ATPase) 6. Cell lysis |
|
|
|
list four conditions that can lead to potassium shift into cells (hypokalemia).
|
1. Insulin (↑ Na/K ATPase)
2. beta-adrenergic agonists (↑ Na/K ATPase) 3. alkalosis (K/H exchanger) 4. hypo-osmolarity |
|
|

questions
|

bold arrows indicate the primary disturbance
|
|
|
|
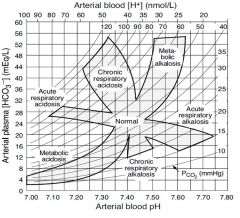
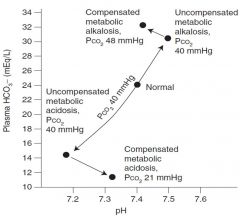
What are the compensatory responses to metabolic acidosis, metabolic alkalosis, respiratory acidosis, and respiratory alkalosis?
|
1. hyperventilation
2. hypoventilation 3. ↑ renal bicarbonate reabsorption 4. ↓ renal bicarbonate reabsorption |
|
|
|
Write the Henderson-Hasselbalch equation as it pertains to determining the pH given bicarbonate concentration and CO2 partial pressure.
|
pH=pKa + log([bicarbonate]/0.03Pco2)
|
|
|
|
What does Winter's formula used for?
|
To determine whether a metabolic acidosis is adequately compensated or if the patient also has a primary respiratory acidosis/alkalosis.
|
|
|
|
What are the three primary systems that regulate acid concentration in body fluids?
|
Lungs (remove CO2=H2CO3), acid/base system, kidneys (remove HCO3)
|
|
|
|
What are the relative rates of action of the acid protective systems?
|
Buffering action seconds
Respiration minutes Kidney hours to days |
|
|
|
What does respiratory alkalosis/acidosis refer to?
|
Changes in PCO2
|
|
|
|
What does metabolic alkalosis/acidosis refer to?
|
Changes in bicarbonate production.
|
|
|
|
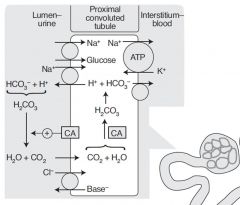
In the kidney, how are bicarbonate and acid eliminated?
|
Bicarbonate is eliminated by filtration, where as H+ is actively secreted. Remember that these processes are tied together by brush border carbonic anhydrase.
|
|
|
|
If a patient is acidemic, how can you distinguish between metabolic vs. respiratory acidosis?
|
Check the PCO2. If higher than 40 mmHg, then respiratory. If lower than 40 mmHg, then metabolic acidosis with compensation.
|
|
|
|
If a metabolic acidosis is identified, what is the next step to identify the cause?
|
Check the anion gap. AG=Na-(Cl+HCO3)
If high, then MUDPILES. If low, then either diarrhea, renal tubular acidosis, or hyperchloremia. |
|
|
|
What are possible causes of high anion gap in metabolic acidosis?
|
MUDPILES: Methanol, Uremia, Diabetic acidosis, Paraldehyde (Phenoformin), Iron (INH), Lactic acidosis (sepsis), Ethylene glycol, Salicylates
|
|
|
|
What are principle causes of respiratory acidosis?
|
Hypoventilation: Obstruction, Lung disease, Opiods, Weak respiratory muscles (MG or Guillain Barre).
|
|
|
|
How do you distinguish between respiratory or metabolic alkalosis?
|
Look at the PCO2, if less than 40 mmHg, then respiratory alkalosis. If greater than 40 mmHg, then metabolic acidosis.
|
|
|
|
If metabolic alkalosis is identified, what are the possible causes?
|
Diuretic use (losing Cl-), vomiting (most common), antacid use, hyperaldosteronism.
|
|
|
|
In metabolic alkalosis, what is the preferable test to do to determine the primary cause?
|
Test urine [Cl-]. If low it means volume depletion.
|
|
|
|
What are the primary causes of respiratory alkalosis?
|
Hyperventilation or aspirin ingestion.
|
|
|
|
What can be used to distinguish between diarrhea and renal tubular acidosis in normal anion gap metabolic acidosis?
|
Look at the urine anion gap. If negative, kidney function is good, indicating diarrhea.
|
|
|
|
What are the three types of renal tubular acidosis?
|
Type 1 (distal), Type 2 (proximal), and Type 4 (hyperkalemic).
|
|
|
|
What is the major problem in type 1 renal tubular acidosis?
|
Defect in the collecting tubule's ability to secrete hydrogen ion. It is associated with hypokalemia and risk for calcium-containing kidney stones.
|
|
|
|
What is the major problem in type 2 renal tubular acidosis?
|
Defect in proximal tubule bicarbonate reabsorption. Associated with hypokalemia and hypophosphatemic rickets. Fanconi Syndrome causes this.
|
|
|
|
What is the major problem in type 4 renal tubular acidosis?
|
Hypoaldosteronism or lack of collecting tubule response to aldosterone. Causes hyperkalemia, inhibition of ammonia secretion in proximal tubule. Causes a drop in urine pH due to decreased buffering capacity.
|
|
|
|
What is the most common type of renal tubular necrosis?
|
Type 4 RTA.
|
|
|
Study
|
Study
|
|
|
Study
|
Study
|
|
|
|
What are the major types of casts that may be found in the urine?
|
RBC casts, WBC casts, Granular (Muddy brown) casts, Waxy casts, Hyaline casts
|
|
|
|
What does the presence of casts indicate?
|
Hematuria or pyuria of renal origin.
|
|
|
|
What would you expect to see in the urine of patients with bladder cancer or kidney stones?
|
RBCs with no casts.
|
|
|
|
What would you expect to see in the urine of patients with cystitis?
|
WBCs with no casts.
|
|
|
|
What disorders commonly present with RBC casts?
|
Glomerulonephritis, ischemia, or malignant HTN.
|
|
|
|
What disorders commonly present with WBC casts?
|
Tubulointerstitial inflammation, acute pyelonephritis, and transplant rejection.
|
|
|
|
What disorder presents with granular or muddy brown casts?
|
Acute tubular necrosis.
|
|
|
|
What would cause the accumulation of waxy casts?
|
Advanced renal disease or chronic renal failure.
|
|
|
|
What are hyaline casts indicative of?
|
They are non-specific.
|
|
|
|
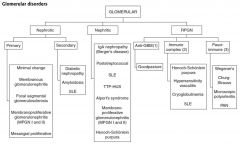
What are the three principle categories of glomerular disorders?
|
Nephrotic, Nephritic, and Rapidly Progressive Glomerulonephritis (RPGN)
|
|
|
Study
|
Study
|
|
|
|
What are the primary modalities for analyzing glomerular disorders?
|
Light microscopy, immunofluorescence microscopy, and electron microscopy.
|
|
|
|
What types of IF microscopy patterns are there?
|
Linear or lumpy bumpy (immune complexes).
|
|
|
|
What is the most common cause of a linear IF pattern?
|
Goodpasture's syndrome which has anti-basement membrane antibodies.
|
|
|
|
What is the definition of Nephritic syndrome?
|
An inflammatory process resulting in HOHA: Hematuria and RBC casts, Oliguria, HTN, Azotemia.
|
|
|
|
What is the definition of Nephrotic syndrome?
|
Massive proteinuria (>3.5 g/day), fatty casts, and edema.
|
|
|
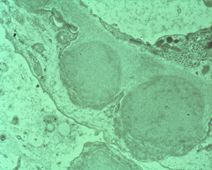
This is an EM of the glomerulus of a patient. What is the diagnosis?
|
Post-streptococcal glomerulonephritis
|
|
|
|
Describe the microscopic characteristics of post-streptococcal glomerulonephritis.
|
LM: enlarged, hypercellular glomeruli with neutrophils and lumpy bumpy appearance.
EM: subepithelial immune complex humps IF: granular |
|
|
|
Who usually gets post-streptococcal GN and how do they present, what is prognosis?
|
Most frequent in children. Peripheral edema and periorbital edema that resolves spontaneously.
|
|
|
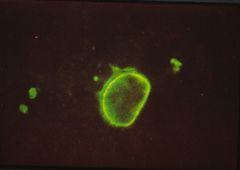
This is an IF stain showing a rim around the nucleus of a cell. What is the diagnosis?
|
Systemic lupus erythematosus.
|
|
|
Who is responsible for ensuring enlisted persons are aware of their right to appeal?
|
Approving athority
|
E4-60
|
|
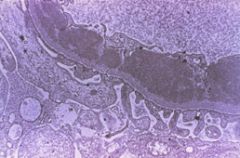
Describe what you see.
|
Subendothelial immune complex deposits. These are DNA-anti-DNA complexes.
|
|
|
|
Why are immune complexes in SLE subendothelial rather than subepithelial?
|
Because they are so large that they can't traverse the basement membrane and get stuck under the endothelial layer.
|
|
|
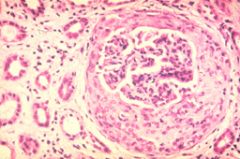
Describe what you see and the syndrome.
|
Infiltration of parietal cells causing a crescent shape. This is rapidly progressive glomerulonephritis and has a poor prognosis.
|
|
|
|
What disease processes may lead to rapidly progressive glomerulonephritis?
|
Goodpasture syndrome, Wegener's granulomatosis, and microscopic polyarteritis.
|
|
|
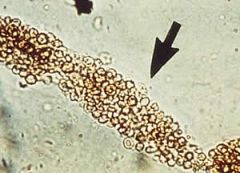
What is this?
|
RBC cast
|
|
|
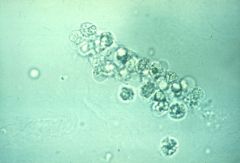
What is this?
|
White blood cell cast
|
|
|
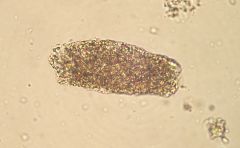
What is this?
|
Granular (muddy brown) cast
|
|
|

What is this?
|
Calcium oxalate crystal
|
|
|
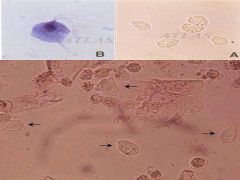
What are these?
|
Trichomonas vaginalis
|
|
|

What is this?
|
Granular (muddy brown) casts
|
|
|

What are these?
|
Triple phosphate crystals (coffin lids).
|
|
|
What is this?
|
Hyaline cast
|
|
|

What are these?
|
Uric Acid
|
|
|
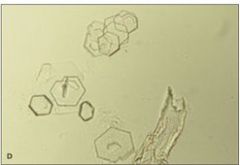
What are these?
|
Cystine cyrstals
|
|
|
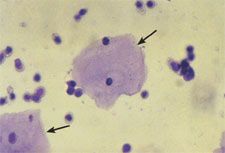
Describe what you see.
|
Squamous epithelial cells and leukocytes.
|
|
|
|
Describe the features of Goodpasture syndrome.
|
It is a type II hypersensitivity with antibodies specific for the glomerular basement membrane. The IF pattern is linear. It is male-dominant, presents with hemauria/hemoptysis b/c of lung involvement.
|
|
|
|
If a patient presents with rapidly progressing glomerulonephritis that is not Goodpasture's syndrome, what test would help distinguish b/n other possibilities?
|
c-ANCA would indicate Wegener's granulomatosis, whereas p-ANCA would indicate Microscopic polyarteritis
|
|
|
|
What are some characteristics of Berger's disease?
|
Increased synthesis of IgA. LM and IF: immune complexes deposit in mesangium. It often presents or flares with URI or acute gastroenteritis.
|
|
|
|
Discuss the major findings of Alport's syndrome.
|
It is caused by a mutation in type IV collagen resulting in a split basement membrane. Associated with ocular disorders and deafness.
|
|
|
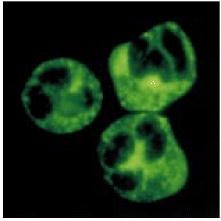
This patient presented with rapidly progressive glomerulonephritis. Shown here is an IF of a neutrophil. What is the diagnosis.
|
Wegener's granulomatosis
|
|
|
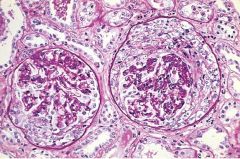
What is this syndrome?
|
Rapidly progressive (crescentic) glomerulonephritis
|
|
|
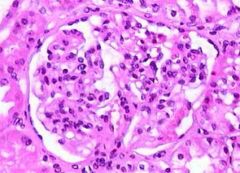
Describe the slide. If IF revealed IgA complexes, what would be the diagnosis?
|
There is mesangial hypercellularity. IgA glomerulopathy (Berger's disease).
|
|
|
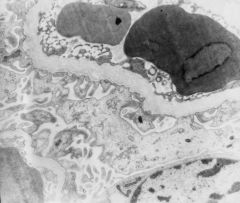
This patient's father and two brothers have kidney problems, but his sisters do not. What is the diagnosis?
|
Alport syndrome
|
|
|
|
What is it called when a patient has pitting edema all over the body?
|
Anasarca
|
|
|
|
On an EM, what is always seen in nephrotic syndrome?
|
Fusion of the podocytes.
|
|
|
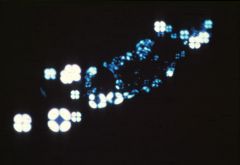
What is this called, and what syndrome is it associated with?
|
Maltese cross, nephrotic syndrome.
|
|
|
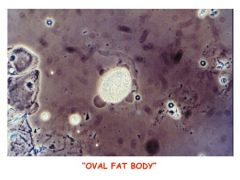
What is this and what syndrome is it associated with?
|
Oval fat body (lipid-engorged macrophage), nephrotic syndrome
|
|
|
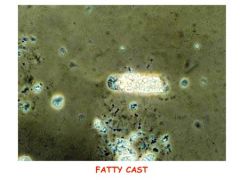
What is this called and what is it associated with? If imaged with birefringence, what would be evident?
|
Fatty cast, nephrotic syndrome. Maltese cross.
|
|
|
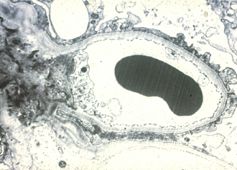
This is the most common form of nephrotic syndrome in children. What would you expect to see in the urine?
|
Elevated albumin because the loss of charge in the basement membrane.
|
|
|
|
Describe minimal change (lipoid nephrosis) disease.
|
It is the most common form of nephrotic disease in children. There is a selective loss of albumin, not globulins due to GBM polyanion loss.
|
|
|
|
What is the most common glomerular disease in HIV patients?
|
Focal segmental glomerulosclerosis.
|
|
|
|
What changes in the glomerulus are seen in focal glomerulosclerosis?
|
Nothing by EM or IF, but LM reveals segmental sclerosis and hyalinosis. May be caused by hyperfiltration.
|
|
|
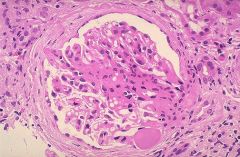
An HIV patient comes in with 3.8 g protein lost in a day. Renal biopsy is performed, what is the diagnosis?
|
Focal segmental glomerulosclerosis
|
|
|
|
What is the most common form of adult nephrotic syndrome?
|
Membranous glomerulonephritis (diffuse membranous glomerulopathy).
|
|
|
|
What would you expect to see on LM, EM, and IF of membranous glomerulonephritis?
|
LM: diffuse capillary and GBM thickening.
EM: spike and dome appearance with subepithelial deposits. IF: granular. SLE's nephrotic presentation |
|
|
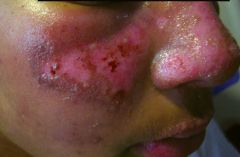
This patients comes in to the hospital and has massive proteinuria. What is the most likely diagnosis?
|
Membranous glomerulonephritis secondary to SLE.
|
|
|
|
What may cause membranous glomerulonephritis?
|
Drugs, infections, SLE, solid tumors.
|
|
|
|
Why are nephrotic syndromes associated with a higher risk of infection?
|
Due to loss of immunoglobulins in the urine.
|
|
|
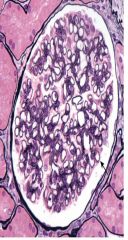
This is an H&E with silver stain. What is the diagnosis?
|
Membranous glomerulonephritis
|
|
|
|
What is the major problem in membranous glomerulonephritis, and how what is its prognosis?
|
Immune complexes depositing in the subepithelial region. It generally has a very bad prognosis, second to rapidly progressive glomerulonephritis.
|
|
|
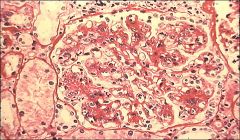
Describe three abnormal changes and list the likely diagnosis.
|
1) large, hypercellular glomerulus
2) mesangial increase 3) PMNs present Membranoproliferative glomerulonephritis |
|
|
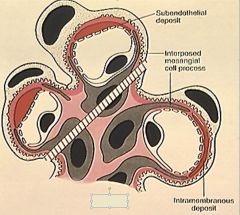
Two different forms of MPGN are indicated here, which is which?
|
Top, Type I. Bottom, Type II
|
|
|
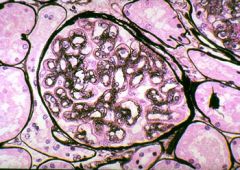
What type of membranoproliferative glomerulonephritity is this? Why?
|
Type I. Tram track appearance due to splitting of the GBM.
|
|
|
|
What are type I and II MPGN associated with?
|
Type I, HBV. Type II, C3 nephritic factor.
|
|
|
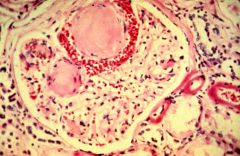
What is the diagnosis?
|
Diabetic nephropathy (Christmas ball disease).
|
|
|
|
What accumulates in the mesangium of patients with diabetic glomerulonephropathy?
|
Type IV collagen.
|
|
|
|
List two major physiological mechanisms for the development of glomerulonephropathy in diabetics.
|
1) Nonenzymatic glycosylation of BM causes loss of charge and proteinuria.
2) NEG of efferent arterioles increases GFR. |
|
|
|
How can you treat diabetic glomerulonephropathy?
|
ACE inhibitor to dilate efferent arterioles (and improve HTN), and better glucose control.
|
|
|
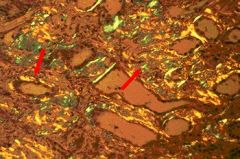
Diagnosis?
|
Based on apple-green birefringence, this is amyloidosis.
|
|
|
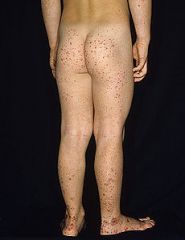
What is this disease, and what kidney disorder may a component of it?
|
Henoch-Schonlein disease
|
|
|
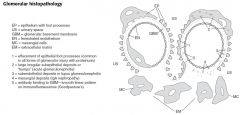
Study
|
Study
|
|
|
|
What are the major complications of kidney stones?
|
Hydronephrosis and pyelonephritis.
|
|
|
|
What are the most common types of kidney stones?
|
Calcium containing crystals: calcium oxalate and calcium phosphate.
|
|
|
|
List four conditions that can cause hypercalcemia.
|
Cancer, ↑PTH, ↑Vitamin D, milk-alkali syndrome.
|
|
|
|
How may calcium oxalate crystals result?
|
Ethylene glycol (antifreeze) or Vitamin C abuse.
|
|
|
|
List two additional names for ammonium magnesium phosphate crystals.
|
Struvite and triple phosphate.
|
|
|
|
What is the second most common kidney stone?
|
Triple phosphate crystals.
|
|
|
|
What causes triple phosphate crystals?
|
Infection with urease-positive bugs (Proteus vulgaris, Staphylococcus, Klebsiella).
|
|
|
|
What my triple phosphate crystals form?
|
Staghorn calculi which can be a nidus for UTIs.
|
|
|
|
What physiological process makes triple phosphate crystals worse?
|
Alkaluria
|
|
|
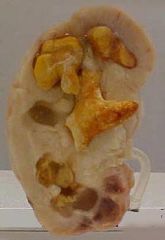
What is this and what causes it?
|
Staghorn calculus caused by triple phosphate crystals.
|
|
|
|
What crystals have a strong association with symptoms of gout?
|
Uric acid crystals resulting from hyperuricemia.
|
|
|
|
What diseases do uric acid crystals often result from?
|
Diseases with high cell turnover, such as leukemia and myeloproliferative disorders.
|
|
|
|
What are cystine crystals typically secondary to?
|
Cystinuria, an autosomal recessive disorder associated with problems in cystine reabsorption.
|
|
|
|
How can cystine crystals be treated?
|
Alkalization of the urine.
|
|
|
|
What is the most common renal malignancy?
|
Renal cell carcinoma.
|
|
|
|
How are creatinine and urea treated in the kidney?
|
Creatinine is filtered, but neither secreted nor reabsorbed. Urea is filtered and reabsorbed.
|
|
|
|
What is azotemia?
|
High BUN.
|
|
|
|
Why does decreased cardiac output lead to azotemia?
|
Because decreased CO leads to decreased GFR which allows more time for proximal tubules to reabsorb urea.
|
|
|
|
What happens to serum creatinine in decreased cardiac output, and how does this compare to the BUN?
|
Decreased GFR will lead to an increase in serum creatinine, but this will not be as severe as for the urea.
|
|
|
|
What BUN/Creatinine ratio indicates pre-renal disease?
|
>15
|
|
|
|
What effect would acute renal failure have on creatinine and BUN?
|
Both will increase proportionately (ratio: 10).
|
|
|
|
What is the most common cause of acute renal failure in the hospital?
|
Acute tubular necrosis.
|
|
|
|
What does ATN result from?
|
Untreated pre-renal failure (decreased CO) leading to decreased renal perfusion and tubular necrosis.
|
|
|
|
Why is ATN fatal if left untreated?
|
The epithelial cells detach from the basement membrane and if the BM is damaged, then there is no place for new cells to attach (irreversible).
|
|
|
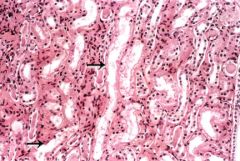
What is the diagnosis?
|
Acute Tubular Necrosis
|
|
|
|
What types of casts would be present in a patient with acute tubular necrosis?
|
Granular (muddy brown) casts.
|
|
|
|
What are the three stages of ATN?
|
1. Inciting event
2. Maintenance (low urine) 3. Recovery (2-3 weeks) |
|
|
|
What is diffuse cortical necrosis?
|
Acute generalized infarction of cortices of both kidneys.
|
|
|
|
How does diffuse cortical necrosis arise?
|
A combination of vasospasm and disseminated intravascular coagulopathy (DIC), obstetric catastrophes such as abruptio placentae, or septic shock.
|
|
|
|
What is drug-induced interstitial nephritis?
|
Acute interstitial renal inflammation resulting from hypersensitivity to drugs.
|
|
|
|
What is typically found in the urine of patients with drug-induced interstitial nephritis?
|
Pyuria (eosinophilia) and azotemia about 1-2 weeks after administration of drug.
|
|
|
|
What symptoms are typically seen in patients with drug-induced interstitial nephritis?
|
Fever, rash, hematuria, and CVA tenderness.
|
|
|
|
What is seen in the urine of patients with acute pyelonephritis?
|
White cell casts.
|
|
|
|
What is the major pathology associated with acute pyelonphritis?
|
It affects the cortex with relative sparing of glomeruli/vessels.
|
|
|
|
What symptoms are typically seen in patients with acute pyelonephritis?
|
Fever, CVA tenderness, nausea, and vomiting.
|
|
|
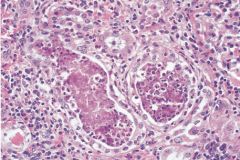
This patient presents with fever, CVA tenderness, nausea, vomiting, and white cell casts. What is the diagnosis?
|
Acute pyelonephritis
|
|
|
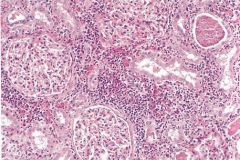
What is the diagnosis?
|
Chronic pyelonephritis
|
|
|
|
Describe findings in chronic pyelonephritis.
|
Coarse, asymetric corticomedullary scarring, blunted calyx. Tubules may contain eosinophilic casts (thyroidization of kidney).
|
|
|
|
List the three major types of kidney tumors.
|
Renal cell carcinoma, Wilm's tumor, and transitional cell carcinoma.
|
|
|
|
What is the most common renal malignancy?
|
Renal cell carcinoma.
|
|
|
|
What is the clinical presentation of renal cell carcinoma?
|
Hematuria, palpable mass, and flank pain.
|
|
|
|
What chromosomal abnormality is associated with renal cell carcinoma?
|
von Hippel-Lindau deletion in chromosome 3.
|
|
|
|
What is the typical course of renal cell carcinoma?
|
Invades the inferior vena cava and then spreads hematogenously. Metastasizes to the lung and bone.
|
|
|
|
What paraneoplastic conditions are associated with renal cell carcinoma?
|
Ectopic EPO, ACTH, PTHrP, prolactin
|
|
|
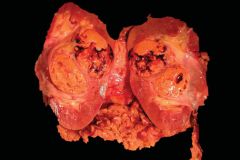
Describe the abnormalities and diagnosis.
|
Renal cell carcinoma. The kidney has been bivalved, revealing a nodular, golden-yellow tumor in the midkidney with areas of hemorrhage and necrosis.
|
|
|
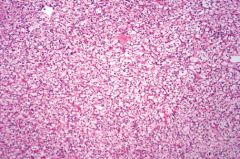
What are these cells and what is the diagnosis?
|
Polygonal clear cells resulting from transformed renal tubule cells. Renal cell carcinoma.
|
|

