![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
|
Cystic Fibrosis |
Cystic fibrosis
* AR inherited condition: 1/2500 Caucasians * Chromosome 7 mutation, multiple abnormalities described * Expressed as defective Cl- channels o High Cl- concentration in secretions, increased viscosity o Sweat Cl- >60 mmol/L o Resultant respiratory, pancreatic, biliary dysfunction Clinical problems * Respiratory o Impaired clearance of secretions, mucociliary dysfunction o Chronic infection, bronchiectasis, dyspnoea, excessive sputum o Air-trapping due to mucous plugging: COAD-like picture o Nasal polyps, chronic sinusitis o Complications + Respiratory failure: cyanosis, CO2 retention + Haemoptysis, pneumothorax, cor pulmonale * GIT o Pancreatic exocrine failure + Malabsorption, malnutrition without enzyme supplementation + Chronic pancreatitis, acute exacerbations + Secondary endocrine failure: diabetes mellitus o Bile secretion impaired + Fat and fat-soluble vitamin malabsorption + Later cirrhosis and portal hypertension o Neonatal meconium ileus * Psychological o Many admissions and procedures o Chronic illness, medicalization o Longer survival resulting in more adult presentations Assessment * Routine, plus: * History related to CF o Admissions, current therapy, respiratory disease, diabetic control o Previous anaesthesia * Examination o General appearance, respiratory, cardiac focus + Signs of respiratory failure, right heart failure * Investigations o CXR, RFT, ABG o FBE, U&E, LFT, glucose o Recent cultures of sputum Optimize * Consult with treating physician to achieve best respiratory function o Bronchodilators, saline nebulisers, antibiotics, physiotherapy Technique * Regional avoids the need for intubation and risk of worsening infection * If GA required by nature of surgery, regional analgesia may result in better * respiratory function postoperatively * Airway o Humidified gases, nebulized saline may be of benefit o Frequent suctioning o Avoid nasal intubation * Ventilation o As for COAD: long I:E, minimize airway pressures, slow rate o Increased FiO2 o Vigilance for pneumothorax * Circulation o Consider invasive monitoring if cor pulmonale: arterial line ± PA catheter * Other considerations o Management of diabetes: fasting, glucose monitoring o Choice of drugs suitable for impaired hepatic function Postoperative * Level of care determined by severity of disease and extent of surgery * Active physiotherapy and early mobilization Smoking |
|
|
Indications and contraindication for TIPS
|
Transjugular Intrahepatic Portosystemic Shunt
INDICATIONS Absolute 1. Multiple episodes of variceal bleeding 2. Refractory variceal hemorrhage despite adequate endoscopic treatment 3. Refractory ascites Relative * Bleeding portal hypertensive gastropathy * Bleeding gastric varices * Gastric antral vascular ectasia * Refractory hepatic hydrothorax * Hepatorenal syndrome * Budd-Chiari syndrome * Veno-occlusive disease * Hepatopulmonary syndrome * Protein-losing enteropathy due to portal hypertension CONTRAINDICATIONS Absolute 1. RHF 2. Severe Encephalopathy 3. Pulmonary hypertension 4. Biliary obstruction 5. Sepsis 6. Hepatic cysts 7. CCF Relative 1. Haematoma 2. Coagulopathy INR >5 3. Thrombocytopenia 4. Hepatocellular carcinoma |
|
|
List the risk factors for venous thromboembolism
|
Patient Factors
* Cancer * Age * Pregnancy * Acute medical ilness * nephrotic syndrome * obesity * previous VTE Drugs * OCP * EPO Surgical * multi trauma * immobility Anaesthetic * CVC |
|
|
VTE risk and treatment
|

|
|
|
Anaesthesia and the elderly
|
CVS
• decreased PNS tone and increased SNS tone • decreased responsiveness of B-receptors (less effect of B-agonists and less HR response to hypotension) • increased SVR (increased arterial rigidity) • increased BP (esp SBP) • increased PAP • myocardial fibrosis & ventricular wall thickening resulting in reduced ventricular compliance • LV strain and hypertrophy • impaired diastolic filling (LV stiffness) • decreased CO and SV at rest (but resting HR unchanged) • impaired baroreceptor responsiveness (due to arterial stiffness) and hence prone to postural hypotension RESPIRATORY • decreased elasticity of chest wall • weakened muscles of respiration (due to loss of muscle mass) • decreased alveolar gas exchange surface • increased dead space (both anatomical and alveolar) • decreased VC, decreased TLC, decreased FEV1 • increased RV but no change in FRC • increased CC so that it encroaches on VT • decreased PaO2 (~100 – age/3) • reduced ventilatory response to hypoxia / hypercapnia • decreased airway protective reflexes increasing the risk of aspiration RENAL • reduced GFR (from 125ml/min to 60ml/min by age 80) mainly due to loss of cortical glomeruli NEURO • decreased number of neurones • decreased numbers of serotonin and dopamine receptors • POCD common • reduced incidence of PDPH • loss of myelin in peripheral nerves, but the PNS is less affected than the CNS THERMOREGULATION • Impaired thermoregulatory ability • Reduced ability to generate heat (less able to vasoconstrict, reduced lean body mass, reduced shivering response) • Body temperature must decrease to a lower level in the elderly before vasoconstriction or shivering is triggered (i.e. not until T = 35.2° compared to 36.1°C in young adults) • decreased BMR OTHER • arthritis almost universal – may lead to positioning difficulty • thin fragile vessel walls may make venous access difficult to achieve • endentulous patients may predispose to difficult mask ventilation • adhesive tapes may damage fragile skin PHARMACOLOGY • Decreased MAC of volatiles (~6% per decade) • reduced dose requirements of all intravenous anaesthetics, opioids and bezodiazepines • increased sensitivity of brain to narcotics • prolonged time to onset (reduced CO) and prolonged duration of action (reduced metabolism) of all NMBD except atracurium & cisatracurium • Reduced Vd (reduced PB and reduced blood volume) hence higher blood levels initially • prolonged arm:brain circulation time • prolonged elimination half-time due to reduced renal clearance & hepatic elimination • increased body fat % resulting in lipid reservoir and prolonged excretion of lipid soluble drugs • lower PB • change in body compartments: loss of skeletal muscle and increased % body fat, reduced blood volume REGIONAL ANAESTHESIA • increased SNS tone at rest hence when decreased by spinal Anaesthesia, greater decreases in BP are seen PAIN • increased pain threshold • less likely to report pain • more likely to suffer adverse effects from NSAIDs (sp gastric & renal) • more sensitive to effects of LA |
|
|
What proportion of anaesthetic agents are involved in periopertive anaphylaxis?
|
60% NMBA
17% Natural rubber latex 15% Antibiotics 4% Colloids 3% Hyponotics 1% Opiods |
|
|
Outline your management of anaphylaxis
|
MANAGEMENT OF ANAPHYLAXIS:
Initial Management: •Call for Help •Stop: exposure to causal Ag, anaesthetic agents, surgery. •ABC’s: ETT, 100% O2, IPPV/PEEP, elevate legs, IV access, IAL/CVC, IDC according to clinical situation •Adrenaline: is the treatment of choice and should be given in all cases of suspected anaphylaxis. Failure to treat anaphylaxis promptly with adrenaline may result in biphasic or protracted anaphylaxis or in a fatal outcome. Note that the dose used initially for hypotension due to anaphylaxis (0.1-1µg/kg) is not the same as in cardiovascular collapse (5-10µg/kg) or cardiac arrest. •Colloids Secondary Therapy: •Hydrocortisone •Antihistamine •Bronchodilators Postoperative Care: •Airway Evaluation: ? delay extubation. •Observation: Observe closely for 8hrs. All cases admitted for 24hrs, HDU/ICU according to severity of anaphylaxis •Continue Mx: adrenaline, hydrocortisone, antihistamine, bronchodilators as indicated. •Investigate: Serum Tryptase •Avoid alcohol, exercise & heat for 24hrs. Discharge & Follow up: •Prednisolone •Oral antihistamines •Refer to allergist for Ix's to determine cause of allergic response: skin testing, RAST •Education: re pptants, avoidance, Mx of attacks. •Information: medical alert card/bracelet/letter •Adrenaline: ‘epipen’ (only if likely to encounter Ag in normal life). Preventative Measures: •PreRx w/ H1/H2 blockers & steroids: ? for IV contrast. •Desensitisation: should be considered where possible •Inhalational & regional anaesthesia. |
|
|
What is the first clinical feature of anaphylaxis?
|
AAGBI Anaphylaxis Guidelines : first clinical feature of anaphylaxis
26% No pulse 24% Difficult to inflate lungs 18% Flush 10% Desaturation 6% Cough 4% Rash 2% ECG abnormality **most common is cardiovascular with bronchospasm and skin changes only slightly less common. |
|
|
What is the main clinical feature of anaphylaxis?
|
AAGBI Anaphylaxis Guidelines
% sole ft worst ft CVS coll 88% 11% 78% Bronchosp 37% 6% 18% Rash 74% Angioede 24% 1% 3% APO 2% 0.3% 0.3% GIT 7% |
|
|
List the ractors which increase the risk of TUR syndrome.
|
Factors which increase the risk of TUR syndrome
* pre-existing hyponatraemia or pulmonary oedema * prostate size larger than 60-100g * inexperienced or slow surgeon * procedures longer than 1 hour * hydrostatic pressure > 60cm H2O (height of bag above patient) * reduced venous pressure (dehydration) * use of large volumes of hypotonic intravenous fluids such as 5% dextrose |
|
|
List the symptoms and signs of TUR Syndrome.
|
8% of cases in a mild form, but is severe in 1-2% of cases.
Resection of prostatic tissue opens an extensive network of venous sinuses, which allows the irrigation fluid to be absorbed into the systemic circulation. 10 to 30mL absorbed per minute of resection time Amounting to up to 1800ml per hour Glycine- containing irrigation solution is slightly hypo-osmotic (200mosm/l) and therefore the classical triad of features that make up TUR syndrome are: 1. Dilutional hyponatraemia. Encephalopathy and seizures may develop when the sodium concentration falls below 120mmol/l. Cerebral oedema may occur. 2. Fluid overload. This causes pulmonary oedema and cardiac failure. 3. Glycine toxicity. This inhibitory neurotransmitter causes depression of the level of consciousness and visual impairment at toxic levels. Symptoms and signs of TUR Syndrome: * tachycardia * nausea and vomiting - caused by hyponatraemia and cerebral oedema * confusion / disorientation - hyponatraemia and cerebral oedema * hypertension (fluid overload), then hypotension (cardiac insufficiency) * transient blindness - glycine toxicity * angina * dyspnoea and hypoxia caused by pulmonary oedema * cardiovascular collapse and arrhythmias (VT/VF) * convulsions * coma (Na <100mmol/l) |
|
|
Assessing Baseline Risk for PONV
|
Incidence
-20-30% of general surgical population -70-80% in high risk patients -0.1-0.2% of day cases will be admitted for PONV Adult Risk Factors Patient -most important independent RF : female, non smoker, history of PONV, motion sickness -also migraine, younger, anxiety, low ASA but no as significant Anaesthetic -volatiles/NO : IMPACT trial, dose related, for every 30minutes increase PONV risk by 60% (eg baseline risk of 10%, but after 30 minutes baseline is 16%) -post op administration of opoids Surgical -association found, but no evidence that there are independent RF -laparoscopy, laparotomy, breast, strabismus, plastic, maxfax, gynae, abdominal MODELS -APFEL et AL -1 point for each : female, non smoker, PONV, opoids 0 - 10%, 1 - 20%, 2 - 40%, 3 - 60%, 4 - 80% Children -risk of POV, nausea is difficult for children to understand -POV risk twice as mush as adults EBERHART et AL 1 point for each -duration > 30 minutes -age > 3 -strabismus -history or family history of PONV SCORE - Baseline Risk 0 - 9% 1 - 10% 2 - 30% 3 - 55% 4 - 70% |
|
|
Describe the model described by Apfel et al
|
Simple Model used to determine baseline risk for PONV in adults
-1 point for each : female, non smoker, PONV, opoids SCORE - Baseline Risk 0 - 10% 1 - 20% 2 - 40% 3 - 60% 4 - 80% |
|
|
Describe the model described by Eberhart et al
|
Simple Model used to determine baseline risk for POV in children
1 point for each -duration > 30 minutes -age > 3 -strabismus -history or family history of PONV SCORE - Baseline Risk 0 - 9% 1 - 10% 2 - 30% 3 - 55% 4 - 70% |
|
|
Methods to reduce base line risk for PONV
|
1. Avoid volatiles
-regional technique IMPACT trial showed a. 59% PONV with volatile or NO anaesthetic b. RR 19% with use of propofol induction c. RR 12% by avoiding NO d. RR 25% with propofol TIVA with Air/O2 2. Avoid NO -causes early PONV, but not late PONV -no effect if baseline risk is low for PONV 3. Minimize Opoids -multi modal -opoid sparing technique -consider adding droperidol 2.5mg per 100mg morphine bag 4. Avoid neostigmine 5. Adequate Hydration O2 therapy has no effect |
|
|
List the procedures associated with gas emoblism?
|
VENOUS
Neurosurgery : craniotomy particular sitting up, Spinal surgery Orthopaedics : Arthroplasty, Arthrography Obstetrics : LSCS, TOP, removal of placenta Head and neck surgery : including laser surgery Intravascular catheters : CVC insertion or disconnection, air adminstration Others : Laparoscopy, Endoscopy, Positive pressure ventilation, hydrogen peroxide ARTERIAL Gas entry directly -Cardiac Surgery -CPB -Angiography -Carotid endarterectomy -Laparoscopy -Decompression sickness Paradoxical air embolism -intracardiac shunt -transpulmonary shunt |
|
|
List the methods of monitoring for the detection of venous air emoblism.
Comment on their sensitivity, specificity, advantages and disadvantages. |
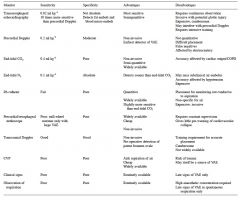
|
|
|
Describe the use of Cell Savers
|
Intraoperative cell salvage includes
-collecting shed blood -concentration -washing Collection -shed blood is obtained from operating site -immediately mixed with anticoagulant (30,000 units heparin per litre of Normal Saline or citrated dextrose) near the suction tip -anticoagulated blood is stored in collection reservoir -120 micron filter removes tissue, clots, orthopaedic cement and other macro debris Concentration -400-700 mls of 'filtered' blood is pumped into spinning centrifuge -concentrates the red cells while removing the plasma and other waste products -plasma overflows from the bowl into the waste bag taking white cells, platelets, free haemoglobin, irrigation fluids, activated clotting factors and cell debris Washing cells -a light sensor detects when centrifuge bowls is full os red cells (225mls with haematocrit above 50%) thereby activating wash cycle -sterile normal saline is pumped into centrifuge bowl, washing the red cells -1-1.5L is used -removing soluable activated clotting factors,proteolytic enzymes, potassium, heparin, red cell debris and free haemoglobin Autologous Transfusion -approximately 50% of shed blood is transfused -haematocrit >50% -reinfused using a 40 micron filter |
|
|
What are the benefits of leucodepletion?
|
Leucodepletion is the removal of white blood cells from a blood component.
Leucodepletion of blood components will result in a reduction in the leucocyte count to less than 1 x 106 white cells per unit. The potential benefits of leucodepletion for patients include: *Reduction in platelet refractoriness * Reduction in febrile non-haemolytic transfusion reactions * Reduction in CMV, EBV, human T cell leukemia virus type 1 transmission risk * Improved chance of finding an organ transplant match if required *Reduction in storage lesion effect *Possible reduction in transfusion associated graft vs host disease (TA-GVHD) risk *Possible reduction in transfusion related immunomodulatory effects, including cancer recurrence, mortality, non-transfusion transmitted infection *possible reduction in postoperative infections * Possible reduction in variant Creutzfeldt-Jakob Disease (vCJD) transmission risk. *potential reduction in reperfusion injury *potential reduction in TRALI |
|
|
What are the benefits of prestorage leucodepletion compared with bedside leucocyte depletion?
|
Leucocyte depletion may be performed before storage (at the blood collection centre) or after storage (in the hospital laboratory or at the bedside).
There is little doubt that the optimum time to remove passenger leucocytes is before storage (called prestorage leucodepletion) for the following reasons: * Better process control and quality assurance Leucocyte filtration is a complicated process that is influenced by factors such as the blood component’s prefiltration cellular composition and plasma content, the temperature of the blood component at the time of filtration, the filtration flow rate, the number of units transfused through the filter, and the timing of the filtration step. Studies have documented a higher incidence of filtration failures when performed at the bedside as compared to leucocyte filtration performed in the laboratory setting. Quality checks and comprehensive quality assurance programs can be more easily performed in the pre-storage setting. Bedside filtration also requires training of many nurses. * Lower incidence of febrile non-haemolytic transfusion reactions (FNHTR) FNHTR are caused not only by leucocyte antigen-antibody reactions but also by the cytokines produced by leucocytes in the transfused blood component. This would be more effectively prevented if the leucocytes were removed immediately after the blood is collected, avoiding the formation of cytokines. This is especially the case with platelet components stored at room temperature as it has been demonstrated that cytokine production occurs more rapidly at 20 °C than 4 °C. * Lower incidence of alloimmunisation and (possibly) diminished immunomodulation that may result from the transfusion of membrane fragments Leucocyte degradation during storage results in cell fragments which may not be removed by post-storage filtration and these can provoke HLA or platelet alloimmunisation. Additionally, it is possible that leucocyte fragments released from cells harbouring leucotrophic viruses may carry such viruses through the filter. * Avoidance or reduction in the incidence of adverse effects directly related to the filtration process Complications such as bradykinin-associated hypotension and transfusion related “red eye” syndrome have been reported with particular types of filters used at the bedside. * Reduction in the incidence of bacterial contamination of blood components Early removal of leucocytes (within 24 hours) may reduce the likelihood of significant bacterial contamination of red cells, particularly relating to Yersinia enterocolica and coagulase-negative Staphylococcus. However, studies relating to bacterial growth in platelet components are much less convincing. |
|
|
Transfusion related Bacterial sepsis
|
Bacterial sepsis is second only to ABO incompatibility as a cause of death from transfusin.
Overall incidence of bacterial contamination ranges from approximately 1:3000–1:1000 units of platelets, and 1 in every 200–600 units of pooled platelets. All platelet components from the Blood Service are screened for bacterial contamination. -sampled 24 hours after collection -screened by bioMerieux BacT/ALERT Automated Microbial Detection System, which uses both aerobic and anaerobic culture bottles. -if positive the bag is recalled and retested (as false positives can occur) -if already transfused, the culture is stained and the patient immediately started on antibiotic prophylaxis -therapeutic management is tailored depending on culture sensitivities and patient clinical condition. |
|
|
Discussion how you would prevent hypoxaemia during OLV
|
OLV used for almost all thoracic operations : lung, esophageal, aortic or mediastinal surgery, to improve surgical access.
Although one lung is ventilated, both lung are perfused. Resulting in -trans pulmonary shunting -impairment of oxygenation -hypoxemia Prevention of Hypoxemia during OLV 1. Improving preoperative lung function -physical therapy -bronchodilators -loosen secretions -treat infections 2. Monitoring DLT placement -DLts are frequently misplaced during placement or dislodged -12% misplaced or dislodged -leading to impaired oxygenation and inadequate lung separation -therefore require frequent fiberoptic to reposition DLT 3. Ventilation strategies -avoid acelectasis in ventilated lung, as it results activation of hypoxic pulmonary vasoconstriction shunting more blood to non ventilated lung, therefore increasing shunt fraction. -avoid increasing PVR in ventilated lung with increasing lung volume (and ventilation pressure), as this ma impede flow to ventilated lung and divert more non ventilated lung -furthermore large volumes over distend alveoli and result in cyclic atelectasis which may result in lung injury (ARDS) -therefore recommend to use moderate tidal volume (6-8ml/kg) and mod PEEP (10mmHg) -however, large tidal volumes (8-15ml/kg) with no PEEP did not show any difference between mod TV and mod PEEP -this area is largely unresolved 4. Oxygen administration to non dependent lung -100% O2 with CPAP 3-10cmH2O -used to prevent and treat hypoxemia -also reducing injury and re-expansion injury -however may disturb surgical field/access 5. Modulation of perfusion -Nitric Oxide is an endothelial dependent vasorelaxing factor -concentration from 5-40 ppm -inhaled, selectively vasodilating the vessels of the ventilated lung, improving oxygenation during OLV 6. Type of Anaesthesia -volatiles inhibit HPV, therefore increase shunt fraction -however, studies are inconclusive -epidural anaesthesia retains HPV, however, studies with epidurals using propofol TIVA with epidural vs GA and epidurals with lower dose GA vs GA alone showed no differences in hypoxaemia. 7. Hemoglobin level -shunt fraction increases and oxygenation decreases with low haemoglobin levels |
|
|
Describe you preoperative evaluation for pneumoneectomy
|
1. Establish diagnosis : most likely bronchogenic carcinoma
-?metastases and their complications : local effects to airway or vascular obstruction -non-metastatic cmanifestations : Eaton Lambert syndrome -other bengings tumors, bronchectasis, and TB 2. Assess respiratory reserve and estimate post-resection lung function a. FEV1 >2L : low risk and require no further testing b. FEV1<2L : predicted post operative-resection FEV1 (ppoFEV1) = multiply preoperative FEV1 by percentage of perfusion to the remaining lung (determined by quantitative lung perfusion scan) c. ppoFEV1>800ml or 40% predicted have an estimated mortality of 15% d. ppoFEV1<800ml : high risk, suggest cardiopulmonary exercise testing e. VO2max<10ml/kg/min are at high risk of death >30% mortality VO2max>15ml/kg/min <15% mortality 3. Surgical risks -Right sided > Left sided (12% cf 3% mortality) -Chest wall resection increase mortality 3 fold -Emergency pneumonectomy for trauma or massive hemoptysis >35% mortality 4. Assess co-morbidities -smoking -airways disease 5. Airway assessment 6. Post operative analgesic plan |
|
|
Describe the anatomical changes to the post-pneumonectomy space following surgical resection.
|
Immediately follow pneumonectomy air fills the space previously occupied by the lung
-no chest tube is placed 1. Decrease in size of PPS 2. Elevation of hemidaigragm 3. Hyperinflation of remaining lung 4. Shift of the mediastinum towards PPS 5. progressive resoprtion of air and replacement with fluid (typically 2 ribs per day, after 2 weeks 80-90% filled with fluid 6. complete opacification of hemithorax by 4 months Other 1. Cardiac -L pneumonectomy : heart rotates counterclockwise -R pneumonectomy : heart shifts into right pleural space 2. Superior displacement of abdominal organs : liver, spleen, stomach Effect of lung function 1. FEV1 and FVC decrease by 50% 2. DCLO is also reduced by 50% but usually normal once corrected for lung volume 3. Lung compliance decrease 4. Aiway resistence increases 5. Dead space increases or decreases 6. ABG (SatO2, PO2, PCO2) at rest do not change, provided there is little or disease in remaining lung Cardiovascular function following pneumonectomy 1. RV EF decreases 20%, LV EF no change 2. As lung as remaining lung is nromal, therefore in no significant change in PA pressures, or CVP |

