![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
83 Cards in this Set
- Front
- Back
|
Unit 5 |
ch 3 |
|
|
Which of the following is a molecular element? |
I |
|
|
Combustion analysis of 63.8 mg of a C, H and O containing compound produced 145.0 mg of CO2 and 59.38 mg of H2O. What is the empirical formula for the compound? |
C3H6O |
|
|
Determine the name for TiCO3. Remember that titanium forms several ions. |
titanium (II) carbonate
|
|
|
What is the charge on the Cr ions in Cr2O3?
|
3+ |
|
|
Give the correct formula for aluminum sulfate.
|
Al2(SO4)3 |
|
|
Give the formula for sulfurous acid. |
H2SO3 |
|
|
Give the name for PBr3.
|
phosphorus tribromide |
|
|
Calculate the molar mass of H2CO3. |
62.03 g/mol |
|
|
Calculate the mass percent composition of sulfur in Al2(SO4)3.
|
21.38 % |
|
|
How many atoms of carbon are contained in 47.6 g of Al2(CO3)3? The molar mass of Al2(CO3)3 is 233.99 g/mol.
|
3.68 × 1023 C atoms |
|
|
How many molecules of butane are contained in 25.0 mL of butane? The density of butane is 0.6011 g/mL and the molar mass is 58.12 g/mol.
|
1.56 × 1023 molecules butane
|
|
|
Determine the empirical formula for a compound that is found to contain 10.15 mg P and 34.85 mg Cl.
|
PCl3 |
|
|
Give a possible molecular formula for C3H5ClO
|
C6H10Cl2O2
|
|
|
What is the chemical formula for iron(III) sulfate?
|
Fe2(SO4)3
|
|
|
The chemical formula for the sulfite ion is
|
S 2- |
|
|
How many moles are there in 3.00 g of ethanol, CH3CH2OH?
|
0.0652 mol |
|
|
Which of the following has the greatest mass?
|
3.88 × 1022 molecules of O2
|
|
|
Which one of the following is not an empirical formula?
|
C2H4O2 |
|
|
Which of the following elements has the least tendency to form an ion?
|
Kr |
|
|
Which of the following is an ionic compound?
|
Mg3(PO4)2 |
|
|
Unit 6 |
ch 9 |
|
|
How many lone pairs of electrons are on the As atom in AsCl3?
|
1 |
|
|
In the best Lewis structure for NO +, what is the formal charge on the N atom?
|
0 |
|
|
Which of the following processes are endothermic?
|
the reaction associated with the ionization energy of potassium.
|
|
|
Use the bond energies provided to estimate ΔH°rxn for the reaction below. |
-128 kJ |
|
|
Use Lewis theory to determine the chemical formula for the compound formed between Al and O.
|
Al2O3 |
|
|
Which of the following reactions is associated with the lattice energy of CaS (ΔH°latt)?
|
Ca2(g) + S2(g) → CaS(s) |
|
|
Place the following in order of decreasing magnitude of lattice energy. |
NaF > KCl > RbBr |
|
|
Choose the compound below that should have the highest melting point according to the ionic bonding model.
|
AlN |
|
|
Give the complete electronic configuration for S2-.
|
1s22s22p63s23p6
|
|
|
Identify the compound with metallic bonding.
|
Li |
|
|
Identify the shortest bond.
|
triple covalent bond
|
|
|
Place the following elements in order of increasing electronegativity. |
Sr < Na < N |
|
|
Which molecule or compound below contains a pure covalent bond?
|
Cl2 |
|
|
Choose the best Lewis structure for BeF2.
|
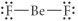
|
|
|
Give the number of valence electrons for ICl5.
|
42 |
|
|
Choose the best Lewis structure for SO42.
|
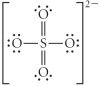
|
|
|
Draw the Lewis structure for CO32- including any valid resonance structures. Which of the following statements is TRUE?
|
The CO32- ion contains two C-O single bonds and one C=O double bond.
|
|
|
Draw the best Lewis structure for the free radical, NO2. What is the formal charge on the N?
|
+1 |
|
|
Which compound has the highest carbon-carbon bond strength?
|
HCCH |
|
|
Place the following in order of increasing bond length. |
NO < NO2 < NO3
|
|
|
Unit 7 |
ch 10 |
|
|
Give the approximate bond angle for a molecule with a trigonal planar shape.
|
120° |
|
|
Using the VSEPR model, the molecular geometry of the central atom in BF3 is __________.
|
trigonal planar |
|
|
Consider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. |
C1 = trigonal planar, C2 = tetrahedral
|
|
|
Place the following in order of decreasing X-A-X bond angle, where A represents the central atom and X represents the outer atoms in each molecule. |
N2O > NO2 > NCl3
|
|
|
How many of the following molecules are polar? |
1 |
|
|
Choose the compound below that contains at least one polar covalent bond, but is nonpolar.
|
CF4 |
|
|
Identify the number of electron groups around a molecule with sp2 hybridization.
|
3 |
|
|
A molecule containing a central atom with sp3d hybridization has a(n) __________ electron geometry.
|
trigonal bipyramidal
|
|
|
Draw the Lewis structure for H3O+. What is the hybridization on the O atom?
|
sp3 |
|
|
Draw the Lewis structure for BrF5. What is the hybridization on the Br atom?
|
sp3d2 |
|
|
How many of the following molecules have sp3d hybridization on the central atom? |
2 |
|
|
List the number of sigma bonds and pi bonds in a single bond.
|
1 sigma, 0 pi
|
|
|
Draw the Lewis structure for the molecule C3H4. How many sigma and pi bonds does it contain?
|
6 sigma, 2 pi
|
|
|
Use the molecular orbital diagram shown to determine which of the following is most stable. |
O22
|
|
|
Draw the molecular orbital diagram shown to determine which of the following is most stable.
|
C22
|
|
|
Using the VSEPR model, the molecular geometry of the central atom in NCl3 is __________.
|
trigonal pyramidal
|
|
|
What are the F-Po-F bond angles in PoF6 ?
|
90° |
|
|
The hybrid orbital set used by the central atom in KrF2 is __________.
|
sp3d
|
|
|
Using the VSEPR model, the electron-domain geometry of the central atom in BrF4- is __________.
|
octahedral |
|
|
Determine the electron geometry (eg) and molecular geometry (mg) of ICl2.
|
eg=trigonal bipyramidal, mg=linear
|
|
|
Unit 8 |
ch 4 |
|
|
Two samples of potassium iodide are decomposed into their constituent elements. The first sample produced 13.0 g of potassium and 42.3 g of iodine. If the second sample produced 24.4 kg of potassium, how many kg of iodine were produced? |
78.4 g |
|
|
How many milliliters of 0.260 M Na2S are needed to react with 40.00 mL of 0.315 M AgNO3? |
48.5 mL
|
|
|
Determine the theoretical yield of HCl if 60.0 g of BCl3 and 37.5 g of H2O are reacted according to the following balanced reaction. A possibly useful molar mass is BCl3 = 117.16 g/mol. |
56.0g HCl |
|
|
Which of the following solutions will have the highest concentration of chloride ions?
|
0.10 M AlCl3 |
|
|
Determine the concentration of a solution prepared by diluting 25.0 mL of a stock 0.188 M Ca(NO3)2 solution to 150.0 mL.
|
0.0313 M
|
|
|
Identify HCl.
|
strong electrolyte, strong acid
|
|
|
Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of H2SO4 and KOH are mixed.
|
H22+(aq) + OH-(aq) → H2(OH)2(l)
|
|
|
What element is undergoing oxidation (if any) in the following reaction? |
C |
|
|
How many grams of NaOH (MW = 40.0) are there in 250.0 mL of a 0.275 M NaOH solution? |
2.75 |
|
|
When 31.2 mL of 0.500 M AgNO3 is added to 25.0 mL of 0.300 M NH4Cl, how many grams of AgCl are formed? |
1.07 g |
|
|
Based on the balanced chemical equation shown below, what volume of 0.250 M K2S2O3(aq), is needed to completely react with 24.88 mL of 0.125 M KI3(aq), according to the chemical equation: |
24.9 mL |
|
|
The mixing of which pair of reactants will result in a precipitation reaction?
|
0.747
|
|
|
What is the oxidation number of the oxygen atom in Na2O2 ?
|
-1 |
|
|
According to the following reaction, what volume of 0.244 M KCl solution is required to react exactly with 50.0 mL of 0.210 M Pb(NO3)2 solution? |
86.1 mL |
|
|
Which of the following pairs of aqueous solutions will form a precipitate when mixed?
|
Hg2(NO3)2 + LiI
|
|
|
The titration of 80.0 mL of an unknown concentration H3PO4 solution requires 126 mL of 0.218 M KOH solution. What is the concentration of the H3PO4 solution (in M)? |
0.114 M |
|
|
What is the concentration (M) of CH3OH in a solution prepared by dissolving 16.8 g of CH3OH in sufficient water to give exactly 230 mL of solution?
|
2.28 |
|
|
What is the concentration of NO3- ions in a solution prepared by dissolving 25.0 g of Ca(NO3)2 in enough water to produce 300. mL of solution?
|
1.02 M |
|
|
How many molecules of HCl are formed when 50.0 g of water reacts according to the following balanced reaction? Assume excess ICl3. |
2.78 × 1024 molecules HCl
|

