![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
Factors associated with the slurry that will influence the filtration process.
|
Liquid properties - Density, viscosity, corrosiveness
Solid properties - Particle shape and particle size distribution. Proportions of solid in the slurry Objective of filtration - collect the solid, liquid or both. Whether the solids have to be washed free from the liquid or solute. |
|
|
Define: Filtration
|
The process of separating, clarifying or harvesting a particular substance from a liquid of gas by means of a porous filter medium that retains solids, but allows the fluid to pass through.
|
|
|
What are the two categories of filtration?
|
1) Simple process - Simple mechanical manipulation for simple solutions and mixtures.
2) Complex process - Formation of 2 phases by the addition of a solid, liquid or gas plus mechanical manipulation. |
|
|
Reasons substances to filter:
|
- Makes an elegant solution
- Removal of potential irritants - Removal of undesired solids present during/after compounding - Recovery of solid - Purifying water for pharmaceutical purposes - Detection/removal or micro-organisms |
|
|
How does a filter media retain solids?
|
- Sieving/straining - Medium has pores smaller than solid (e.g. cellulose acetate)
- Impingement - Matrix of random fibres traps solids and retains them (medium is thick and often used from removing solids from gases) - Attractive forces - Electrostatic forces retains solids of medium - Autofiltration - Retentant residue acts as a second, more effective filter medium |
|
|
Filter aids:
|
INSOLUBLE absorbent powders added to certain liquids and the filter medium prior to filtration.
Reduces filter pore clogging by increasing the permeability of the cake, decreasing the compressibility of the cake and absorbing insoluble oils. |
|
|
Darcy's equation:
|
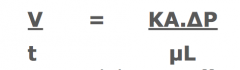
|
|
|
Filtration Rate depends on -
|
u - Viscosity of slurry
^P - Pressure difference across L - Thickness of filter medium + deposited cake A - Area available for filtration K - Permeability co-efficient based on the nature of solid and filter medium . ...a large K value = a high porosity and filtration rate. Influenced by S/A, particle size and distribution of solids in slurry. |
|
|
Factors influencing filtration rate -
|
- Broad particle size distribution - solids of dissimilar size and shape - large particles form interstices that soon clog up with smaller particles.
- Narrow particle distribution with large size particles - form large pores, rapid rate of filtration, paper needs to be strong - Narrow particle distribution with small particle size - Reduces filtration rate, increases efficiency. |
|
|
Filter media will be chosen due to:
|
- Chemical nature of the solvent
- Volume to be filtered - Pressure - Degree of filtration needed - Product viscosity - Filtration temp. |
|
|
** Increasing filtration rate **
|
- Increasing area available for filtration (fluted filter paper)
- Increasing pressure difference across the filter cake (syringe or vacuum) - Decreasing filtrate viscosity - Decreasing thickness of filter cake - Increasing permeability of cake (filter aid) |
|
|
Centrifugation -
|
Used for separation of a solid/liquid or liquid/liquid. Used when filtration is difficult (e.g. slimy compressible substances). Solution is placed in a rapidly rotating basket.
|
|
|
Clarification -
|
Separation without use of filters. e.g. temperature, gravity, sedimentation.
|
|
|
Colation/Straining
|
Crude filtration process where liquid is poured through a porous medium (like a cloth).
|
|
|
Decantation -
|
Pouring off top layer of liquid where a sediment has settled.
|
|
|
Displacement washing -
|
Soluble impurities removed from insoluble liquid by adding a washing solvent and then decanting it off.
|

