![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
51 Cards in this Set
- Front
- Back
|
What is the mobile phase in GC? |
Inert gas (Ne, N, Ar) |
|
|
What sort of compounds is GC used for? |
Volatile organic compounds |
|
|
How does the mobile phase in GC differ to other types of chromatography? |
It does not interact with the analyte, its only function is to transport it through the column. |
|
|
What are the two types of GC? |
1. Gas-solid chromatography 2. Gas-liquid chromatography |
|
|
What is the stationary phase in GC? |
A solid or a liquid coated onto a solid support |
|
|
In GC, what are the two ways of structuring the stationary phase? |
1. Packed into a 'column' (column chromatography) 2. Coated onto the inner walls of a very narrow capillary (open tubular chromatography, capillary GC) |
|
|
What is the term for the mobile phase in GC? |
Carrier gas / Eluent gas |
|
|
When was the first commercial apparatus for GC introduced to the market? |
1955 |
|
|
Approximately how many gas chromatographs are currently used throughout the world? |
200,000 |
|
|
Outline the general process of GC |
1. Sample is vaporised and injected onto the head of the chromatographic column 2. Sample is transporter through the column by the flow of inert, gaseous mobile phase 3. The column contains a liquid/solid stationary phase which is adsorbed onto the surface of an inert solid |
|
|
Name four similarities between GC and HPLC |
1. Flowing mobile phase 2. Injection port 3. Separation column 4. Detector |
|
|
What is the rate of diffusion in liquid chromatography, compared to the rate of diffusion in GC? |
Much slower (liquid vs gas) |
|
|
The theory for what kind of chromatography was developed from the theory for GC? |
HPLC |
|
|
Name two requirements for a sample in GC |
1. Must be volatile 2. Must be stable at operating temperature |
|
|
What five factors does separation in GC depend on? |
1. Polarity of stationary phase (higher D for stationary phase = increased retention. Large differences in D for solute mix = increased separation.) 2. Temperature (higher temp = higher proportion of solute in gas phase = lower retention. Large diff in BP for solute mix = increased separation) 3. Flow rate of carrier gas (increased flow = decreased retention = decreased efficiency = decreased separation) 4. Amount of sample injected (overloading = decreased separation) 5. Length of the column (longer = better separation) |
|
|
Name eight applications of GC in pharmaceutical science |
1. Quality control 2. Quantification of bulk drug in preparations 3. Stability studies 4. Pharmacokinetic studies 5. Drug application studies 6. Pharmacognosy 7. Checking suitability of packaging materials 8. Detection of counterfeit drugs |
|
|
What are the five classical GC components? |
1. Mobile phase (carrier gas) reservoir 2. Sample introduction device 3. GC separation column 4. Detector 5. Data logger/computer |
|
|
Draw a basic schematic of a GC instrument |
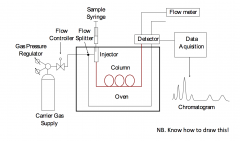
|
|
|
State the pressure and flow rate of the carrier gas in GC |
1. Supplied at 10-40psi 2. Flow at 2-120ml/min |
|
|
What is the main factor controlling the rate of movement along a column? |
Gas temperature |
|
|
What are two minor factors contributing to the rate of movement along a column? |
1. Gas viscosity 2. Gas density (high density = faster analysis) |
|
|
How should the sample be introduced in GC? |
As a narrow band. Liquids through a self-sealing rubber septum. Gases via a large volume gas-tight syringe or gas sampling valve. |
|
|
What is split injection used for? |
As a means of reducing sample volume for open tubular columns |
|
|
What percentage of injected sample reaches the column in split injection? |
0.1-10% |
|
|
How does split injection work? |
1. The sample is injected through the septum into the sample evaporisation zone 2. At the split point, some of the sample enters the chromatography column and the remainder goes through the split needle valve to a waste vent 3. Split ratios: 20:1-500:1 |
|
|
State the two major designs of column ovens |
1. Vertical mounting 2. Horizontal mounting |
|
|
State two desirable properties fro a column oven |
1. Easily accessible 2. Fast heat-up AND cool-down |
|
|
What is the temperature range of a column oven? |
-50C - 450C |
|
|
What are five advantages of temperature programming? |
1. Shorter run time 2. Sharper peaks 3. Increased signal-to-noise ratio 4. Optimised resolution and k 5. Useful for mixtures with broad boiling point range |
|
|
What are the two types of column format in GC? |
1. Packed column - spherical particles. 2. Open tubular (OT) - mostly a coated inner wall of a tube/capillary |
|
|
What is the structure, length, internal diameter and advantage of a packed column? |
1-6m length. 2-4mm internal diameter. Made of glass, copper or stainless steel. Poor efficiency but cheaper than capillaries. |
|
|
What is the structure, length, internal diameter and advantage of an open tubular column? |
10-100m length. 0.1-0.75mm internal diameter. Made of open tubular fused silica (high quality quartz), coated with polyimide or aluminium. Capillary columns are less robust than packed columns, but working life can be extended by bonding stationary phase onto inner walls of column. |
|
|
What are the three types of capillary columns? |
1. WCOT (liquid coated) 2. SCOT (solid support coated) 3. PLOT (porous solid support is SP) |
|
|
State two types of solid support in GLC with examples |
1. Silica based (diatomaceous earth, silanised glass beads, glass capillary wall) 2. Polymeric sorbents (porous polystyrene, PTFE for very polar compounds) |
|
|
State the most commonly used liquid stationary phase in GC |
dimethylpolysiloxane (DMS) (non-polar) |
|
|
How can dimethylpolysiloxane (DMS) be made more polar? |
Replace dimethyl groups with polar groups (e.g. phenyl groups, cyano groups, amine groups. Polyglycol phases for very polar) |
|
|
What interactions are present in non-polar columns (like DMS)? |
1. van der Waals forces predominate 2. No hydrogen bonding or ionic interactions 3. Substance separate in order of increasing boiling point (i.e. more volatile substances elute first) |
|
|
What interactions are present in more polar columns (like DMS/phenyl; DMS/cyano)? |
1. Van der Waals still occur 2. But depending on degree of cross-functionalisation: emerging interactiosn occur which will influence separation (dipole-dipole; dipole-induced dipole; pi-pi interactions). 3. Substances still tend to separate in order of increasing BP but some substances will elute later than would be predicted from volatility alone. |
|
|
What interactions are present in very polar columns (like polyglycol phases)? |
1. Hydrogen bonding can occur 2. Polar substances of similar BP to non-polar substances will have longer retention times. 3. There will still be van der Waals interactions between non-polar regions of SP and non-polar analytes. |
|
|
What is the process of choosing a stationary phase in GC? |
1. If unsure about sample content, begin development phase with DMS. 2. Low bleed columns are more temperature stable. 3. Use least polar phase which results in adequate separation and time (non-polar phase = longer life) 4. If targeted analysis, choose phase with similar polarity 5. agilent.com has column selection guide 6. check the literature: someone has likely already done this |
|
|
What polarity solvents are most suitable for GC? |
Generally non-polar/medium polarity compounds. |
|
|
State five common derivatisation reactions |
1. Alkylation - addition of an alkyl group 2. Arylation - addition of an aromatic functionality 3. Silylation - replacement of acidic hydrogen on compound with an alkylsilyl group like SiMe3 4. Acylation - addition of an -RC=O group 5. Esterification - Conversion of an organic acid to an ester by reaction with alcohol |
|
|
What is the purpose of derivatisation? |
To enhance volatility/stability of high polarity or thermally labile compounds |
|
|
What are the two groups of detectors in GC? |
1. Concentration dependant detectors 2. Mass-flow dependant detectors |
|
|
Which group of detectors in GC do not usually destroy the sample? |
Concentration dependant detectors |
|
|
Which group of detectors in GC usually destroy the sample? |
Mass flow dependant detectors |
|
|
State three GC detectors and their type |
1. Flame ionization (FID) - Mass flow 2. Thermal conductivity (TCD) - concentration 3. Electron capture (ECD) - concentration |
|
|
State eight ideal detector characteristics |
1. Adequate sensitivity 2. Good stability and reproducibility 3. Linear response 4. Operable temperature range for GC applications 5. Short response time not limited by flow rate 6. High reliability 7. Similarity in response for solutes 8. Non-destructive of sample |
|
|
How does a flame ionisation detector (FID) work? |
1. Sample is pyrolysed in hydrogen flame to produce electrons (jet) 2. Potential difference of 400V put across the flame and resulting current is measured. |
|
|
How does a thermal conductivity detector (TCD) work? |
1. Presence of electrically heated element whose resistance at constant electrical power depends on thermal conductivity of surrounding gas 2. TC of He or N2 gases is higher than most organic compounds (~6 fold). Detection is based on drop in TC by eluting solute molecules 3. Reference cell and measurement cell format based on wheatstone bridge circuity (channel 1 - carrier gas only, channel 2 - carrier gas + analytes) |
|
|
How does a mass spectrometric detector (MSD) work? |
1. Detects analytes eluting from colun based on mass-to-charge ration 2. Measurement requires analytes to be ionised and in gas phase (GC applicability, LC-MS available) 3. Most common ionisation sources in GC are electron impact and chemical ionisation |

